United States Non-Invasive Prenatal Testing Market Size and Share Analysis - Growth Trends and Forecast Report 2025-2033
Buy NowUnited States Non-Invasive Prenatal Testing Market Size & Forecast 2025-2033
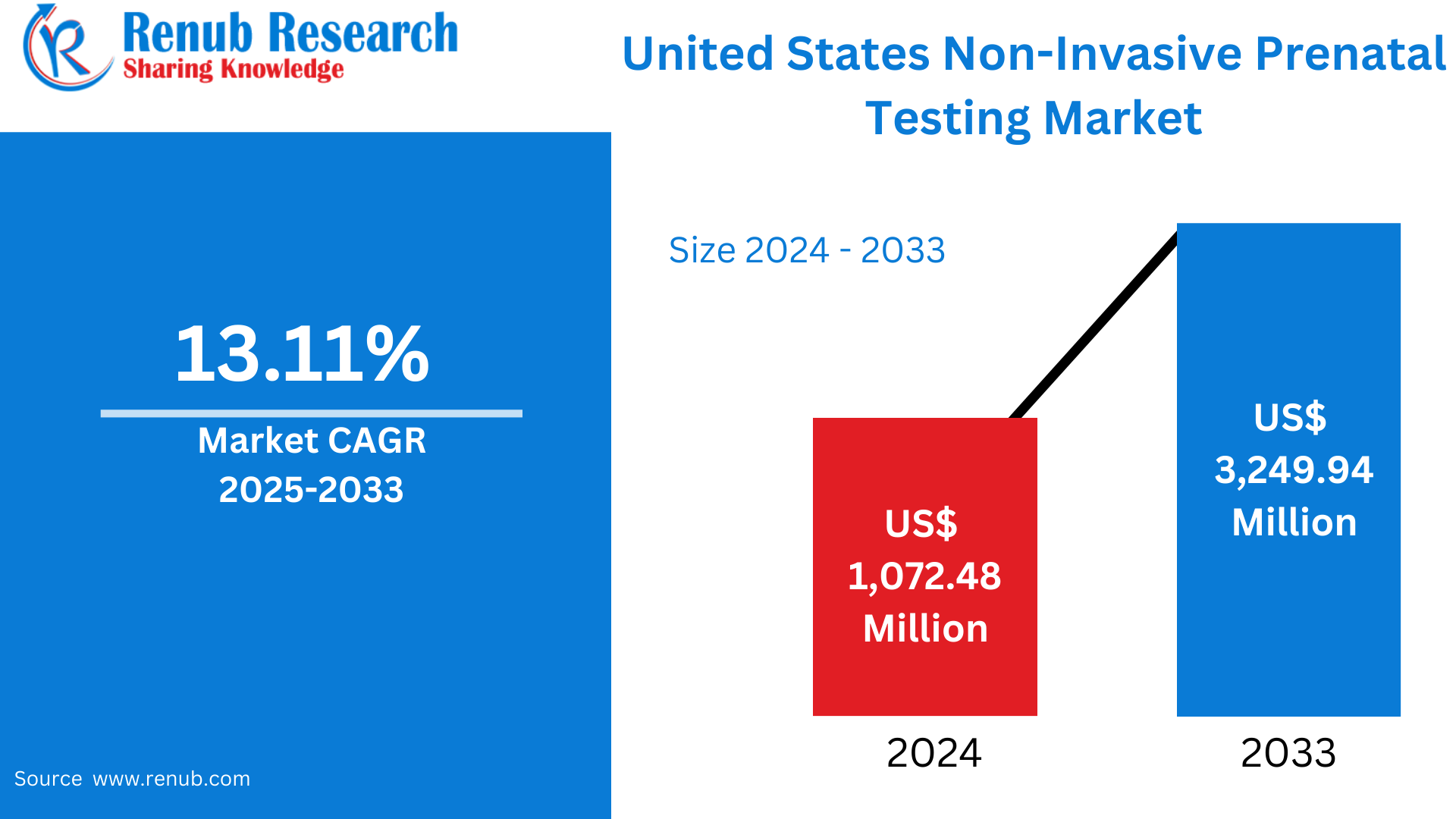
United States Non-Invasive Prenatal Testing Market is expected to reach US$ 3,249.94 million by 2033 from US$ 1,072.48 million in 2024, with a CAGR of 13.11% from 2025 to 2033. The safety of non-invasive techniques, more awareness, better insurance coverage, technological improvements, and an increase in genetic diseases are the main factors propelling the non-invasive prenatal testing market in the United States.
The report United States Non-Invasive Prenatal Testing Market & Forecast covers by Component (Instruments, Kits and Reagents, Services), Application (Down Syndrome (Trisomy 21), Edwards Syndrome (Trisomy 18), Patau Syndrome (Trisomy 13), Turner Syndrome, Other Applications), End User (Hospitals, Diagnostic Labs) and Company Analysis, 2025-2033.
United States Non-Invasive Prenatal Testing Market Overview
Technological developments and growing medical community acceptability have propelled the non-invasive prenatal testing (NIPT) sector in the United States to tremendous expansion. NIPT provides early and precise screening for chromosomal disorders such Down syndrome, trisomy 18, and trisomy 13 by analyzing fetal DNA using a mother's blood sample. This technique has become more and more popular since, in contrast to more conventional invasive procedures like amniocentesis, it is non-invasive and does not endanger the fetus. Furthermore, advancements in next-generation sequencing technology have increased NIPT's precision and reach, making it possible to identify a greater variety of genetic abnormalities, such as single-gene illnesses and microdeletions.
The business has also profited from rising knowledge of NIPT's benefits among pregnant women and healthcare professionals. Regardless of age or risk factors, NIPT is now advised as a first-line screening option for all pregnant women by professional groups such as the American College of Obstetricians and Gynecologists (ACOG). Furthermore, NIPT is now more widely available to a larger population, which lessens the financial strain on patients, thanks to the expansion of insurance coverage, including Medicaid and commercial insurers. The growing frequency of genetic abnormalities and the growing need for safer, more accurate prenatal testing alternatives are thus likely to fuel the industry's continued fast expansion.
A higher number of Down syndrome cases and pregnant women's growing knowledge of NIPT are two major factors driving the expansion of the Down syndrome sector. The market is expected to expand as the number of instances of Down syndrome rises. According to a 2023 study by the National Center on Birth Defects and Developmental Disabilities, Down syndrome is still the most common chromosomal abnormality in the US. About 6,000 babies in the US are identified with Down syndrome each year, which equates to about 1 in 700 births. As a result, the demand for prenatal testing rises due to the growing number of instances of Down syndrome, which drives sector growth throughout the projection period.
Growth Drivers for the United States Non-Invasive Prenatal Testing Market
Improved Reimbursement Policies
A larger population may now more easily get non-invasive prenatal testing (NIPT) thanks in large part to improved funding regulations. Many pregnant moms now find NIPT to be an economical alternative because to the large reduction in out-of-pocket expenses brought about by expanded insurance coverage, including Medicaid and commercial insurers. NIPT's usage was formerly restricted to higher-risk pregnancies due to its expense, which was a hurdle for some. But now that coverage has been increased, more women may use this safer, more precise screening technique, irrespective of their age or risk factors. In addition to raising adoption rates, this change is helping identify genetic disorders early on, which improves prenatal care and results. As insurers continue to see the benefits of NIPT, the trend is anticipated to continue.
Increased Awareness and Recommendations
Non-invasive prenatal testing (NIPT) uptake has been further accelerated by increased knowledge and updated professional organization guidelines. Regardless of age or risk factors, the American College of Obstetricians and Gynecologists (ACOG) now advises NIPT as a first-line screening option for all expectant mothers. This change is a result of increased awareness of NIPT's precision, security, and capacity to identify chromosome disorders including Down syndrome, trisomy 18, and trisomy 13 earlier in pregnancy. As awareness has grown, NIPT is now seen as a routine option for all pregnant moms, when previously it was only available for higher-risk pregnancies. The approach has become more generally accepted as a reliable prenatal screening method as a result of these recommendations changes.
Safety and Non-Invasiveness
The safety and non-invasiveness of non-invasive prenatal testing (NIPT) are two of its main benefits. NIPT uses a straightforward blood test from the mother and poses no danger to the fetus, in contrast to conventional prenatal screening techniques like amniocentesis or chorionic villus sampling (CVS), which have a slight chance of miscarriage. This makes NIPT a desirable choice for pregnant parents who value safety but also want precise findings for genetic disorders like as trisomy 18, trisomy 13, and Down syndrome. Many women choose NIPT since it is non-invasive, particularly those who are pregnant or who are worried about the hazards associated with more intrusive testing techniques. Consequently, NIPT has grown in popularity as a screening method in prenatal treatment.
Challenges in the United States Non-Invasive Prenatal Testing Market
Regulatory Oversight and False Results
The absence of FDA supervision is a major obstacle in the non-invasive prenatal testing (NIPT) business in the United States. The accuracy and dependability of NIPT tests are questioned since they are laboratory-developed and not subject to FDA approval. Even while NIPT is usually thought to be quite reliable, false positives can happen and cause pregnant parents’ needless worry. These erroneous findings might occasionally lead to decisions—like ending a pregnancy—being made based on false information. The tests are not subject to the same stringent clearance procedures as other medical devices because to the lack of FDA oversight, which raises questions about their reliability and the possible repercussions of inaccurate findings. Resolving this issue is essential to preserving confidence and enhancing results.
Ethical and Social Implications
Significant ethical and societal issues are brought up by the growing use of non-invasive prenatal testing (NIPT), especially in relation to selective abortion and the possibility of eugenics. Pregnancy termination decisions may be influenced by NIPT as it enables the early discovery of genetic disorders, particularly if parents decide to end the pregnancy due to perceived hereditary features or limitations. This brings up ethical concerns regarding the worth of life and the possibility of prejudice against people with particular illnesses. Furthermore, deciphering genetic data can be difficult, and many parents experience mental turmoil while dealing with the findings. The social arguments around genetic screening and the psychological effects of these choices underscore the necessity of careful consideration of the moral ramifications of the extensive use of NIPT in prenatal treatment.
Non-invasive Prenatal Testing Market News
May 2024: A new cfDNA-based fetal RhD test was introduced by Natera, Inc., a world leader in cell-free DNA (cfDNA) and genetic testing. Given the severe lack of Rho(D) immune globulin treatment (RhIg) in the US, this introduction is well-timed for the healthcare industry as it helps doctors treat patients. Using blood samples from pregnant women, Natera's test determines the fetal RhD status and may be performed as early as nine weeks into gestation. Analysis of complex pseudogene and RhD-CE-D hybrid variants is part of this.
United States Non-Invasive Prenatal Testing Market Segmentation
Component
- Instruments
- Kits and Reagents
- Services
Application
- Down Syndrome (Trisomy 21)
- Edwards Syndrome (Trisomy 18)
- Patau Syndrome (Trisomy 13)
- Turner Syndrome
- Other Applications
End User
- Hospitals
- Diagnostic Labs
All the Key players have been covered
- Overview
- Key Persons
- Recent Development & Strategies
- Financial Insights
Company Analysis:
- Eurofins Scientific
- F. Hoffmann-La Roche Ltd
- Invitae Corporation
- Illumina Inc.
- Natera Inc.
- Centogene NV
- Qiagen
Report Details:
| Report Features | Details |
| Base Year |
2024 |
| Historical Period |
2021 - 2024 |
| Forecast Period |
2025 - 2033 |
| Market |
US$ Million |
| Segment Covered |
Component, Application and End User |
| Application Covered |
|
| Companies Covered |
|
| Customization Scope |
20% Free Customization |
| Post-Sale Analyst Support |
1 Year (52 Weeks) |
| Delivery Format |
PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on request) |
Customization Services available
- Analysis of Market Size and Its Segments
- More Company Profiles (Upto 10 without any additional cost):
- Additional Countries (Other than mentioned Countries):
- Region/Country Specific Reports:
- Market Entry Strategy:
- Region-Specific Market Dynamics:
- Regional Market Share Analysis:
- Trade Analysis:
- Production Insights:
- Others Customized Requests:
For more information contact our analysts.
Need More Assistance?
- Talk to our analysts to get more precious information on the current market trends.
- Include more countries and segments and customize the report based on the final requirement.
- Get a competitive advantage in your industry by knowing the report findings and making a positive impact on your revenues and operations.
- Our analysts are always ready to provide more help and pertinent information if you need any additional assistance.
1. Introduction
2. Research Methodology
2.1 Data Source
2.1.1 Primary Sources
2.1.2 Secondary Sources
2.2 Research Approach
2.2.1 Top-Down Approach
2.2.2 Bottom-Up Approach
2.3 Forecast Projection Methodology
3. Executive Summary
4. Market Dynamics
4.1 Growth Drivers
4.2 Challenges
5. United States Non-Invasive Prenatal Testing Market
6. Market Share Analysis
6.1 Component
6.2 Application
6.3 End User
7. Component
7.1 Instruments
7.2 Kits and Reagents
7.3 Services
8. Application
8.1 Down Syndrome (Trisomy 21)
8.2 Edwards Syndrome (Trisomy 18)
8.3 Patau Syndrome (Trisomy 13)
8.4 Turner Syndrome
8.5 Other Applications
9. End User
9.1 Hospitals
9.2 Diagnostic Labs
10. Porter’s Five Analysis
10.1 Bargaining Power of Buyers
10.2 Bargaining Power of Suppliers
10.3 Degree of Rivalry
10.4 Threat of New Entrants
10.5 Threat of Substitutes
11. SWOT Analysis
11.1 Strength
11.2 Weakness
11.3 Opportunity
11.4 Threat
12. Key Players Analysis
12.1 Eurofins Scientific
12.1.1 Overview
12.1.2 Key Persons
12.1.3 Recent Development & Strategies
12.1.4 Financial Insight
12.2 F. Hoffmann-La Roche Ltd
12.2.1 Overview
12.2.2 Key Persons
12.2.3 Recent Development & Strategies
12.2.4 Financial Insight
12.3 Invitae Corporation
12.3.1 Overview
12.3.2 Key Persons
12.3.3 Recent Development & Strategies
12.3.4 Financial Insight
12.4 Illumina Inc.
12.4.1 Overview
12.4.2 Key Persons
12.4.3 Recent Development & Strategies
12.4.4 Financial Insight
12.5 Natera Inc.
12.5.1 Overview
12.5.2 Key Persons
12.5.3 Recent Development & Strategies
12.5.4 Financial Insight
12.6 Centogene NV
12.6.1 Overview
12.6.2 Key Persons
12.6.3 Recent Development & Strategies
12.6.4 Financial Insight
12.7 Qiagen
12.7.1 Overview
12.7.2 Key Persons
12.7.3 Recent Development & Strategies
12.7.4 Financial Insight
Reach out to us
Call us on
USA: +1-478-202-3244
INDIA: +91-120-421-9822
Drop us an email at
info@renub.com