Human Papilloma Virus Testing Market Size and Share Analysis - Growth Trends and Forecast Report 2025-2033
Buy NowHuman Papilloma Virus (HPV) Testing Market Trends & Summary
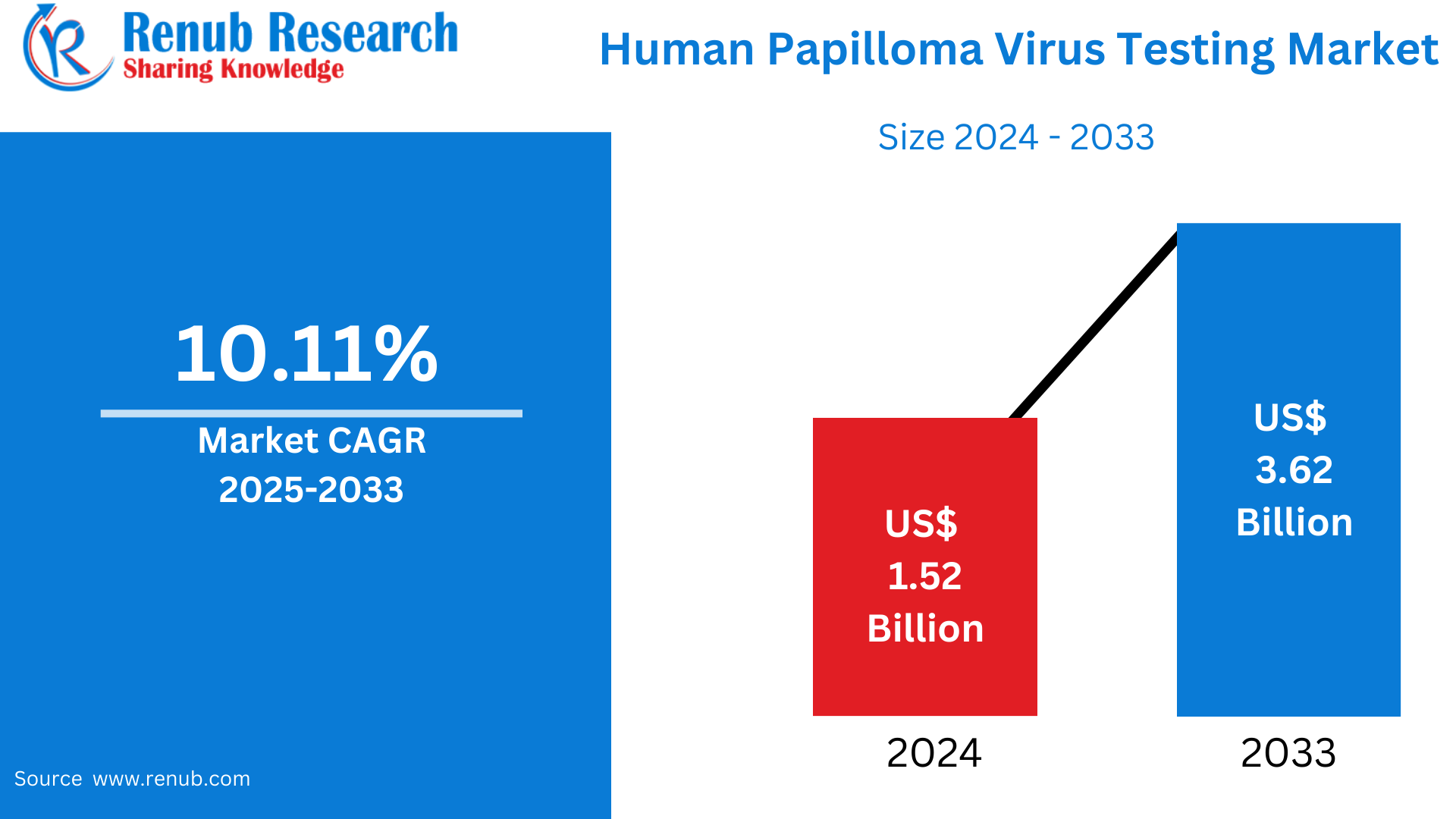
The Human papillomavirus (HPV) Testing Market was USD 1.52 billion in 2024 and is expected to reach USD 3.62 billion by 2033, with a CAGR of 10.11% from 2025 to 2033. The growth is due to increasing awareness of cervical cancer, increased screening programs, and technological advancements in diagnostic technologies in global healthcare systems.
The report Human Papilloma Virus (HPV) Testing Market & Forecast covers by Application (Cervical Cancer Screening, Vaginal Cancer Screening), Product (Consumables, Instruments, Services), Technology (Polymerase chain reaction (PCR), Liquid-based cytology, Immunodiagnostics, Hybrid Capture, Others), End Use (Hospitals & Clinics, Laboratories, Others), Countries and Company analysis 2025-2033.
Human Papilloma Virus (HPV) Testing Market Outlooks
Human Papilloma Virus (HPV) testing is a clinical diagnostic test conducted to identify the existence of HPV DNA or RNA in cells, mostly from the cervix. HPV is an epidemic sexually transmitted infection and some high-risk types are recognized to be causative for cervical cancer, as well as other anogenital and oropharyngeal cancers. HPV testing is generally done in regular cervical cancer screenings, typically at the same time or after a Pap test. It allows identifying women who are at risk prior to cell abnormality occurrence so that it may be prevented or treated early.
Internationally, the use of HPV testing is increasing with growing awareness regarding prevention of cervical cancer, government-sponsored screening programs, and WHO's initiative to eliminate cervical cancer as a public health issue. Developed countries have embraced HPV-based screening on a large scale, and developing nations are also adopting it, with the support of global health programs. Its high sensitivity and long-term risk prediction make it a method of choice in contemporary gynecological practice.
Growth Driver in the Human Papilloma Virus (HPV) Testing Market
Increasing Awareness and Government Screening Programs
International awareness programs and country-level screening programs have increased demand for HPV testing to a large extent. Governments in developed and emerging economies are adopting routine cervical cancer screening guidelines for women aged 30 and older. WHO organizations advocate for HPV testing as a key tool for early detection of cancer. Greater awareness of HPV-associated cancers and immunization also reinforces demand. These efforts account for early diagnosis, prompt intervention, and overall reduction in HPV-associated disease burden, thus driving the market forward. SEP 2024, The United States, Australia, India, and Japan are rolling out a key initiative to fight cervical cancer across the Indo-Pacific, a preventable disease which is a significant health crisis in the region. This initiative is part of larger announcements made during the Quad Leaders Summit.
Technological Breakthroughs in HPV Testing
Innovation in diagnostic methods—like PCR, next-generation sequencing, and self-sampling kits—has revolutionized HPV testing by enhancing accuracy, accessibility, and convenience. Automated platforms, multiplex assays, and liquid-based cytology enable quicker turnaround times and large-scale screening. These technologies are particularly precious in resource-poor settings and enable wider adoption in public health systems. Ongoing R&D spending by diagnostic firms serves to improve test sensitivity and affordability, fueling additional market growth. June 2023, The WHO has prequalified a fourth test for human papillomavirus (HPV). Although most HPV infections will clear on their own, a few of the higher-risk types can cause cervical cancer, so HPV testing is important for cervical cancer screening.
Rising Prevalence of HPV-Associated Cancers
Growing global incidence of HPV-related cancers, particularly cervical cancer, is a strong market driver. Cervical cancer is the fourth leading female cancer in the world according to the WHO, and the overwhelming majority of cases are caused by high-risk HPV types. Growing cases of cancer have driven the need for early detection strategies, putting HPV testing at the center of preventive medicine. As healthcare professionals and patients strive for surefire diagnostic measures, HPV testing is also becoming a necessity in women's health care. An estimated 47,984 new cases of cancer occur each year in the United States in areas of the body where human papillomavirus (HPV) commonly occurs.
Challenge in the Human Papilloma Virus (HPV) Testing Market
Limited Access in Low-Income Countries
In spite of worldwide awareness, HPV testing is not readily available in low- and middle-income countries because of a lack of healthcare infrastructure and resources. There are few laboratory facilities, skilled professionals, and regular screening programs in most areas. Cultural taboo and sexual stigma also discourage HPV testing participation. This inequality avoids early diagnosis and enhances the risk of cervical cancer at advanced stages. It is essential to overcome these barriers using cost-effective point-of-care diagnostics and education initiatives for equitable market expansion.
Exorbitant Cost of Sophisticated HPV Testing Technologies
The price of advanced HPV testing technologies, including PCR-based platforms and DNA sequencing, can be out of reach for healthcare systems and some patients. As developed nations implement these technologies into regular screening, the affordability gap restricts accessibility in resource-scarce settings. In addition, maintenance costs, quality assurance, and specialized staff requirements contribute to higher operating costs for clinics and laboratories. This cost factor hinders mass screening activities, particularly in developing countries.
Human Papilloma Virus Cervical Cancer Testing Market
Cervical cancer testing is the leading segment in the HPV testing market. Almost all cervical cancers are associated with persistent infection with high-risk HPV types, so early detection is essential. Governments and NGOs are encouraging HPV-based cervical cancer screening to supplement or replace conventional Pap smears. Incorporation of HPV DNA testing in primary screening algorithms enhances detection rates and long-term surveillance. This trend has raised the demand for dependable, sensitive, and scalable testing solutions, considerably boosting the cervical cancer testing market.
Consumables Human Papilloma Virus Testing Market
Consumables such as reagents, assay kits, swabs, and sample collection devices make up a crucial part of the HPV testing market. With reoccurring testing and increasing use of self-sampling kits, the need for superior consumables is increasing consistently. Diagnostic clinics and laboratories depend on regular supply and compatibility with multiple automated platforms. Additionally, innovation in multiplex testing and sample preservation has resulted in the creation of sophisticated consumables, providing improved stability and accuracy. This consumables segment provides recurring revenue, supporting market growth.
Immunodiagnostics Human Papilloma Virus Testing Market
Immunodiagnostics HPV tests identify particular viral proteins or host immune reactions through antibody-antigen interactions. These assays are simpler than molecular methods and are commonly employed in screening programs, especially in resource-constrained environments. They represent a cost-saving alternative for identifying high-risk HPV types and early cervical lesions. While less sensitive than PCR, continued advances in immunoassay design and the discovery of biomarkers are improving reliability. This section is important in making HPV testing available in various healthcare settings.
Polymerase Chain Reaction (PCR) Human Papilloma Virus Testing Market
PCR-based HPV tests are considered the gold standard for high-risk HPV detection based on their enhanced sensitivity and specificity. Multiple HPV genotypes can be identified with these tests in a single assay, which facilitates accurate risk stratification and monitoring. Increasing clinical use of real-time PCR and multiplex platforms has driven the market strongly. Automation and cost-saving measures are rendering PCR testing more viable in large-scale screening programs. With precision diagnostics becoming more prominent, PCR continues to be a mainstay in HPV testing.
Human Papilloma Virus Testing Laboratories Market
Test-based HPV laboratory testing leads the market with high-volume, high-precision, scalable offerings of centralized laboratories. The laboratories provide standardized test protocols to hospitals, public health programs, and private clinics. Diagnostic laboratories are embracing automation, high-capacity platforms, and LIMS integration as demand for cervical cancer screening increases. Reference laboratories and outsourcing services have led to strong, long-term growth for this market segment.
United States Human Papilloma Virus Testing Market
The U.S. market for HPV testing is developed and ongoing because of extensive awareness, national screening initiatives, and high usage of sophisticated diagnostics. Routine screening for HPV in women is advised by the CDC, and the services are usually covered by insurers. Technological advancements, robust regulatory backing, and dominance of major players make the U.S. a focal point for HPV diagnostics. Demand for home and self-collection kits is also increasing, thus fueling diversification in the market.
France Human Papilloma Virus Testing Market
In France, the HPV testing market is growing steadily with the integration of HPV screening into the national health system. Since 2020, HPV testing has been recommended as the primary screening method for women aged 30 and above, replacing the Pap smear. The French government subsidizes screening, raising participation and test volumes. With a solid public health infrastructure and improving awareness, the market is moving toward automated molecular testing platforms and large-scale laboratory operations.
India Human Papilloma Virus Testing Market
India's HPV testing market is in the growth stage, fueled by rising awareness and pilot screening across different states. Adoption is restricted to urban areas and private healthcare due to cost and infrastructure reasons. Government and NGOs are enforcing broader accessibility through low-cost testing solutions and mobile health vans. With a heavy load of cervical cancer, India has huge long-term potential, particularly as domestic manufacturers and public policy reduce costs.
Saudi Arabia Human Papilloma Virus Testing Market
The Saudi Arabian market for HPV testing is growing as the government continues to focus more on women's health and prevention of cancer. The Ministry of Health is initiating HPV vaccination and screening programs, particularly in private clinics and urban hospitals. Despite initial resistance from cultural sensitivity, awareness campaigns and education are slowly enhancing test acceptance. With a young, technologically savvy population and continued healthcare reform under Vision 2030, Saudi Arabia has the potential to become a dominant force in regional HPV diagnostics market.
Human Papilloma Virus Testing Market Segmentation
Application
- Cervical Cancer Screening
- Vaginal Cancer Screening
Product
- Consumables
- Instruments
- Services
Technology
- Polymerase chain reaction (PCR)
- Liquid-based cytology
- Immunodiagnostics
- Hybrid Capture
- Others
End Use
- Hospitals & Clinics
- Laboratories
- Others
Countries
North America
- United States
- Canada
Europe
- France
- Germany
- Italy
- Spain
- United Kingdom
- Belgium
- Netherlands
- Turkey
Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Malaysia
- Indonesia
- Australia
- New Zealand
Latin America
- Brazil
- Mexico
- Argentina
Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
All companies have been covered
- Overview
- Key Persons
- Recent Development & Strategies
- Financial Insights
Company Analysis
- Abbott Laboratories
- BioMerieux SA
- Bio-Rad Laboratories, Inc.
- Epigenomics AG
- Siemens Healthineers AG
- Hologic Inc
- Qiagen NV
- F. Hoffmann-La Roche Ltd
Report Details:
| Report Features | Details |
| Base Year |
2024 |
| Historical Period |
2021 - 2024 |
| Forecast Period |
2025 - 2033 |
| Market |
US$ Billion |
| Segment Covered |
Application, Product, Technology, End User and Countries |
| Countries Covered |
|
| Companies Covered |
|
| Customization Scope |
20% Free Customization |
| Post-Sale Analyst Support |
1 Year (52 Weeks) |
| Delivery Format |
PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on request) |
Customization Services available
- Analysis of Market Size and Its Segments
- More Company Profiles (Upto 10 without any additional cost):
- Additional Countries (Other than mentioned Countries):
- Region/Country Specific Reports:
- Market Entry Strategy:
- Region-Specific Market Dynamics:
- Regional Market Share Analysis:
- Trade Analysis:
- Production Insights:
- Others Customized Requests:
For more information contact our analysts.
Need More Assistance?
- Talk to our analysts to get more precious information on the current market trends.
- Include more countries and segments and customize the report based on the final requirement.
- Get a competitive advantage in your industry by knowing the report findings and making a positive impact on your revenues and operations.
- Our analysts are always ready to provide more help and pertinent information if you need any additional assistance.
1. Introduction
2. Research Methodology
2.1 Data Source
2.1.1 Primary Sources
2.1.2 Secondary Sources
2.2 Research Approach
2.2.1 Top-Down Approach
2.2.2 Bottom-Up Approach
2.3 Forecast Projection Methodology
3. Executive Summary
4. Market Dynamics
4.1 Growth Drivers
4.2 Challenges
5. Human Papilloma Virus Testing Market
5.1 Historical Market Trends
5.2 Market Forecast
6. Human Papilloma Virus Testing Market Share Analysis
6.1 By Application
6.2 By Product
6.3 By Technology
6.4 By End Use
6.5 By Countries
7. Application
7.1 Cervical Cancer Screening
7.2 Vaginal Cancer Screening
8. Product
8.1 Consumables
8.2 Instruments
8.3 Services
9. Technology
9.1 Polymerase chain reaction (PCR)
9.2 Liquid-based cytology
9.3 Immunodiagnostics
9.4 Hybrid Capture
9.5 Others
10. End Use
10.1 Hospitals & Clinics
10.2 Laboratories
10.3 Others
11. Countries
11.1 North America
11.1.1 United States
11.1.2 Canada
11.2 Europe
11.2.1 France
11.2.2 Germany
11.2.3 Italy
11.2.4 Spain
11.2.5 United Kingdom
11.2.6 Belgium
11.2.7 Netherlands
11.2.8 Turkey
11.3 Asia Pacific
11.3.1 China
11.3.2 Japan
11.3.3 India
11.3.4 South Korea
11.3.5 Thailand
11.3.6 Malaysia
11.3.7 Indonesia
11.3.8 Australia
11.3.9 New Zealand
11.4 Latin America
11.4.1 Brazil
11.4.2 Mexico
11.4.3 Argentina
11.5 Middle East & Africa
11.5.1 Saudi Arabia
11.5.2 UAE
11.5.3 South Africa
12. Porter’s Five Forces Analysis
12.1 Bargaining Power of Buyers
12.2 Bargaining Power of Suppliers
12.3 Degree of Rivalry
12.4 Threat of New Entrants
12.5 Threat of Substitutes
13. SWOT Analysis
13.1 Strength
13.2 Weakness
13.3 Opportunity
13.4 Threat
14. Key Players Analysis
14.1 Abbott Laboratories
14.1.1 Overview
14.1.2 Key Persons
14.1.3 Recent Development & Strategies
14.1.4 Revenue Analysis
14.2 BioMerieux SA
14.2.1 Overview
14.2.2 Key Persons
14.2.3 Recent Development & Strategies
14.2.4 Revenue Analysis
14.3 Bio-Rad Laboratories, Inc.
14.3.1 Overview
14.3.2 Key Persons
14.3.3 Recent Development & Strategies
14.3.4 Revenue Analysis
14.4 Epigenomics AG
14.4.1 Overview
14.4.2 Key Persons
14.4.3 Recent Development & Strategies
14.4.4 Revenue Analysis
14.5 Siemens Healthineers AG
14.5.1 Overview
14.5.2 Key Persons
14.5.3 Recent Development & Strategies
14.5.4 Revenue Analysis
14.6 Hologic Inc
14.6.1 Overview
14.6.2 Key Persons
14.6.3 Recent Development & Strategies
14.6.4 Revenue Analysis
14.7 Qiagen NV
14.7.1 Overview
14.7.2 Key Persons
14.7.3 Recent Development & Strategies
14.7.4 Revenue Analysis
14.8 F. Hoffmann-La Roche Ltd
14.8.1 Overview
14.8.2 Key Persons
14.8.3 Recent Development & Strategies
14.8.4 Revenue Analysis
Reach out to us
Call us on
USA: +1-478-202-3244
INDIA: +91-120-421-9822
Drop us an email at
info@renub.com