Europe Cardiac Surgical Devices Market – Innovations & Forecast 2025–2033
Buy NowEurope Cardiac Surgical Device Market Size and Forecast 2025-2033
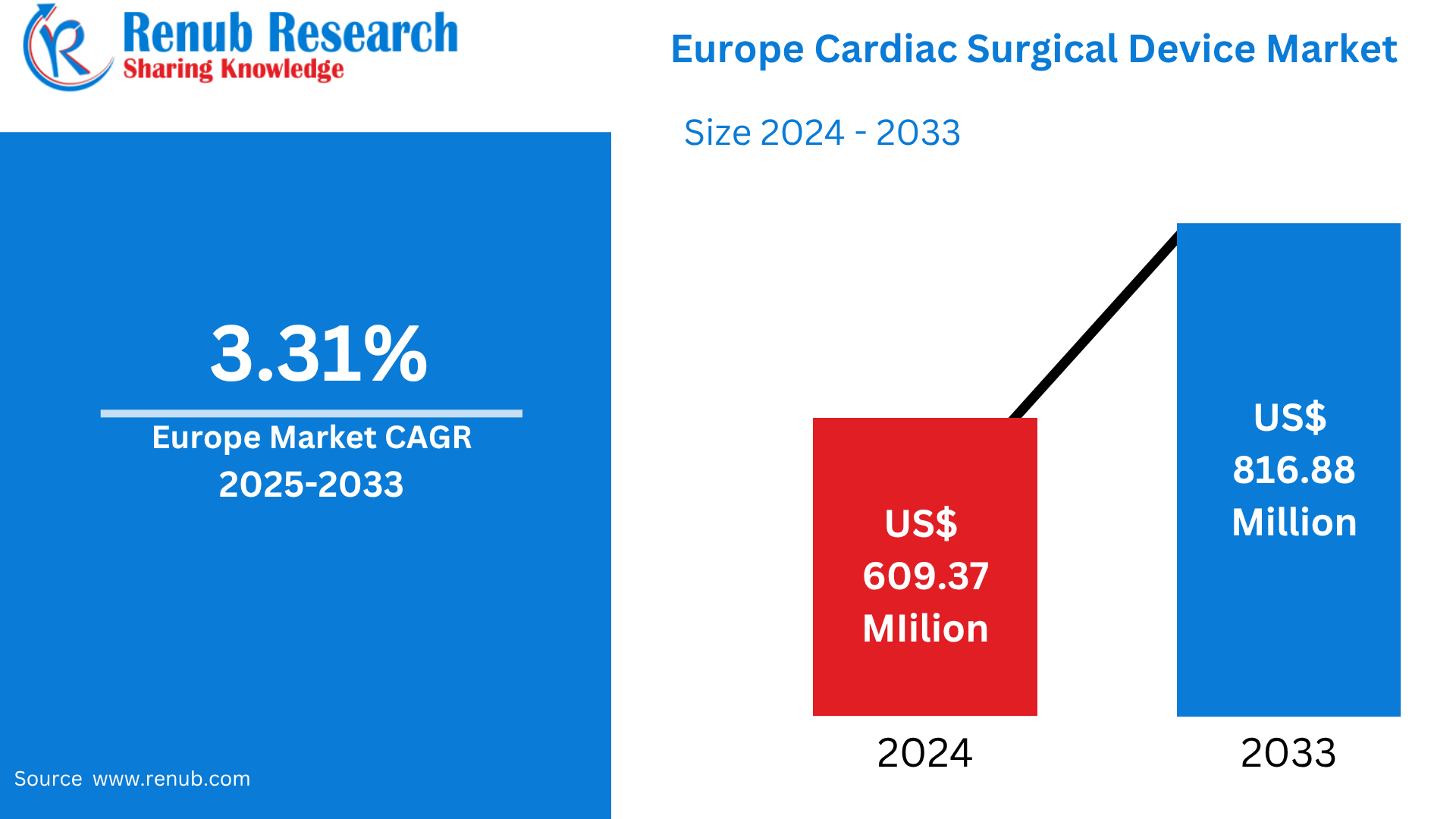
Europe Cardiac Surgical Device Market is expected to reach US$ 816.88 million by 2033 from US$ 609.37 million in 2024, with a CAGR of 3.31% from 2025 to 2033. An aging population, rising incidences of cardiovascular disease, advancements in technology, growing medical expenditure, demand for less invasive procedures, improved diagnostic techniques, favorable government policies, increasing awareness among people about cardiac health, are the key drivers of the European cardiac surgical device market.
Europe Cardiac Surgical Device Market Report by Product Type (Beating Heart Surgery Systems, Cardiopulmonary Bypass Equipment, Cardiac Ablation Devices, Perfusion Disposable), Application (Congential Heart Defects, Cardiac Arrthymia, Coronary Heart Disease, Congestive Heart Failure, Others), End User (Hospitals, Ambulatory Surgical Centers, Others), Countries and Company Analysis 2025-2033.
Europe Cardiac Surgical Device Market Overview
Specialized health equipment and devices referred to as cardiac surgical devices are employed during cardiac operations to diagnose, manage, or repair cardiovascular disease. These include catheters, valves, grafts, retractors, heart-lung machines, and assistive devices like ventricular assist devices (VADs). They find use in procedures such as heart transplantation, valve replacement, coronary artery bypass grafting (CABG), and minimally invasive cardiac surgery. These devices encourage surgical precision, stabilize cardiac function, and maintain circulation. They are constructed with safety and accuracy in mind. Newer cardiac surgical devices have improved performance with the help of technological advancements, which enhances the outcome for patients and accelerates recovery post-surgery.
Several significant factors are fueling the cardiac surgery device market in Europe. Demand for surgeries is significantly boosted by an older population and the increasing rate of cardiovascular diseases. Technology advancements like AI integration and minimally invasive techniques enhance the efficacy and outcome of surgery. The market is sustained by improved healthcare infrastructure and increased healthcare expenditure in European countries. Growth is also facilitated by government initiatives on early detection and therapy of cardiac ailments. The Europe cardiac surgical device market is also being fueled by heightened heart health awareness, a high concentration of the industry's leading players, and the growing requirement for complex, patient-specific surgical technologies.
Growth Drivers for the Europe Cardiac Surgical Device Market
Technological Advancements
The provision of cardiac treatment is being radically revolutionized by technological innovations, which act as the primary growth driver of the European cardiac surgical device market. The accuracy, efficiency, and safety of cardiac procedures are being improved through the integration of artificial intelligence (AI), robot-assisted surgery, and advanced imaging technology. Advances make it possible to perform less invasive procedures, reduce recovery times for patients, and improve clinical outcomes. Medtronic established the benchmark for similar innovation in Europe in May 2024 by deploying AI on every Reveal LINQTM Insertable Cardiac Monitor (ICM) device in the U.S., Australia, and New Zealand through a cloud-based upgrade. Additionally, to enhance diagnostic accuracy, GE HealthCare launched the RevolutionTM Vibe CT system in April 2025. The system comes with AI-enabled solutions and Unlimited One-Beat Cardiac imaging. These findings are expected to drive the adoption of advanced cardiac surgical equipment across Europe, fueling market growth and setting the standard for patient care.
Rising Cardiovascular Disease Prevalence
The European market for cardiac surgical equipment is expanding rapidly as a result of increasing prevalence of cardiovascular disease (CVD). The demand for advanced surgical interventions is increasing due to the increasing incidence of heart conditions like arrhythmias, heart failure, and coronary artery disease. The British Heart Foundation (BHF) 2022 report notes that a minimum of 150,000 individuals in the UK had vascular dementia in 2022 and this number is expected to rise to 350,000 by the year 2030. The demand for more effective surgical interventions is emphasized by the increase in cardiovascular-related conditions. The demand for cardiac surgical equipment, including stents, valves, and diagnostic devices, is being fueled by the growing incidence of heart ailments across Europe. Demand for advanced surgical equipment is rising as an increasing number of patients need cardiac treatment, ensuring enhanced outcomes and faster patient recovery.
Improved Healthcare Infrastructure
Demand for cardiac surgical equipment in Europe is largely fueled by enhanced healthcare infrastructure. The presence of experienced medical professionals, cardiac specialized centers, and advanced hospital facilities has made high-quality cardiac care accessible. The investments by European countries in advanced medical technology are spurring the utilization of advanced cardiac surgery equipment. Patient volumes for cardiac surgery are increasing due to greater service accessibility and the expansion of healthcare infrastructure in urban and rural areas. In addition, well-established healthcare infrastructures promote timely interventions, sophisticated post-operative management, and early diagnosis—all of which drive the growing demand for innovative and effective cardiac devices. The cardiac surgery technology marketplace is propelled by this infrastructural development that also ensures improved patient outcomes.
Challenges in the Europe Cardiac Surgical Device Market
High Costs
The market for cardiac surgical devices in Europe is severely hampered by high prices. Advanced technology, like AI-powered gadgets and robotic surgical systems, are frequently costly, which restricts their accessibility, especially in smaller healthcare facilities or nations with tighter finances. The hefty initial investment and ongoing maintenance expenses may discourage adoption, even though these technologies provide better results and efficiency. The widespread adoption of these technologies is further hampered by the fact that not all healthcare systems adequately reimburse them. This cost barrier hinders the uptake of innovative gadgets, especially in areas with tight healthcare budgets or unstable economies.
Regulatory Hurdles
The European market for cardiac surgical devices is severely hampered by regulatory barriers. The lengthy and intricate clearance process for medical equipment necessitates adherence to stringent EU rules and guidelines. Prior to being released into the market, devices must pass stringent testing, clinical trials, and certifications like the CE mark. These regulations can raise development costs, restrict smaller businesses' access to markets, and postpone the adoption of innovative technologies. Furthermore, producers may find it challenging to stay up to date with market expectations and compliance due to the uncertainty created by ever changing rules.
France Cardiac Surgical Device Market
An aging population, an increase in the prevalence of cardiovascular disorders, and technological breakthroughs in medicine are all contributing to the substantial expansion of the French cardiac surgical device market. Robotic-assisted surgeries and bioresorbable stents, which improve surgical accuracy and patient recovery periods, are examples of the minimally invasive techniques that are becoming more popular in France. The industry is still seeing innovation from major international firms like Medtronic, Abbott Laboratories, and Boston Scientific as well as from regional firms like Getinge AB and LivaNova PLC. However, obstacles such as the expensive price of sophisticated equipment and the intricate regulatory framework of the EU Medical Device Regulation (MDR) may prevent wider use. Notwithstanding these obstacles, the industry is well-positioned for future expansion thanks to developments in technology and rising demand for efficient cardiac care.
Germany Cardiac Surgical Device Market
Due to the aging of the population, rising rates of cardiovascular illness, and improvements in medical technology, the German cardiac surgical device market is expanding significantly. One of the main factors is the growing popularity of minimally invasive procedures, which have advantages including shorter recovery periods and less complications. Further driving growth are technological advancements like AI-enhanced diagnostic tools and robotic-assisted procedures. Additionally, the extensive use of cutting-edge cardiac surgical devices is supported by Germany's established healthcare system and advantageous reimbursement regulations. However, obstacles like expensive devices and complicated regulations could hinder expansion. Resolving these problems will be essential to maintaining the industry's growing trajectory.
United Kingdom Cardiac Surgical Device Market
The aging population, rising prevalence of cardiovascular illness, and improvements in medical technology are all contributing to the strong growth of the cardiac surgical device market in the United Kingdom. One of the main factors is the growing popularity of minimally invasive procedures, which have advantages including shorter recovery periods and less complications. Further driving growth are technological advancements like AI-enhanced diagnostic tools and robotic-assisted procedures. Furthermore, the extensive use of cutting-edge cardiac surgical devices is supported by the UK's established healthcare system and advantageous payment regulations.
Spain Cardiac Surgical Device Market
Spain's cardiac surgical device business is witnessing substantial expansion, driven by an aging population, rising frequency of cardiovascular disorders, and breakthroughs in medical technology. The advent of minimally invasive techniques, such as Transcatheter Aortic Valve Replacement (TAVR) and Percutaneous Coronary Intervention (PCI), has reduced the need for open-heart surgeries, contributing to shorter recovery periods and improved patient outcomes. Further driving growth are technological advancements like AI-enhanced diagnostic tools and robotic-assisted procedures. Advanced cardiac surgical equipment are widely adopted in Spain thanks to the country's well-established healthcare system and advantageous payment rules. However, obstacles like expensive devices and complicated regulations could hinder expansion. Resolving these problems will be essential to maintaining the industry's growing trajectory.
Europe Cardiac Surgical Device Market Segments:
Product Type
- Beating Heart Surgery Systems
- Cardiopulmonary Bypass Equipment
- Cardiac Ablation Devices
- Perfusion Disposable
Application
- Congential Heart Defects
- Cardiac Arrthymia
- Coronary Heart Disease
- Congestive Heart Failure
- Others
End Users
- Hospitals
- Ambulatory Surgical Centers
- Others
Country
- France
- Germany
- Italy
- Spain
- United Kingdom
- Belgium
- Netherlands
- Russia
- Poland
- Greece
- Norway
- Romania
- Portugal
- Rest of Europe
All companies have been covered from 4 viewpoints:
- Company Overview
- Key Persons
- Recent Development & Strategies
- Sales Analysis
Key Players Analysis
- Abbott Laboratories
- Boston Scientific Corporation
- Edwards Lifesciences
- Cardinal Health Inc.
- Medtronic PLC
- GE Healthcare
- LivaNova PLC
- Terumo Corporation
Report Details:
| Report Features | Details |
| Base Year |
2024 |
| Historical Period |
2021- 2024 |
| Forecast Period |
2025 - 2033 |
| Market |
US$ Million |
| Segment Covered |
By Product Type, By Application, By End User and By Countries |
| Countries Covered |
|
| Companies Covered |
|
| Customization Scope |
20% Free Customization |
| Post-Sale Analyst Support |
1 Year (52 Weeks) |
| Delivery Format |
PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on request) |
1. Introduction
2. Research & Methodology
2.1 Data Source
2.1.1 Primary Sources
2.1.2 Secondary Sources
2.2 Research Approach
2.2.1 Top-Down Approach
2.2.2 Bottom-Up Approach
2.3 Forecast Projection Methodology
3. Executive Summary
4. Market Dynamics
4.1 Growth Drivers
4.2 Challenges
5. Europe Cardiac Surgical Devices Market
5.1 Historical Market Trends
5.2 Market Forecast
6. Market Share Analysis
6.1 By Product Type
6.2 By Application
6.3 By End User
6.4 By Countries
7. Product Type
7.1 Beating Heart Surgery Systems
7.2 Cardiopulmonary Bypass Equipment
7.3 Cardiac Ablation Devices
7.4 Perfusion Disposable
8. Application
8.1 Congential Heart Defects
8.2 Cardiac Arrthymia
8.3 Coronary Heart Disease
8.4 Congestive Heart Failure
8.5 Others
9. End User
9.1 Hospitals
9.2 Ambulatory Surgical Centers
9.3 Others
10. Countries
10.1 France
10.2 Germany
10.3 Italy
10.4 Spain
10.5 United Kingdom
10.6 Belgium
10.7 Netherlands
10.8 Russia
10.9 Poland
10.10 Greece
10.11 Norway
10.12 Romania
10.13 Portugal
10.14 Rest of Europe
11. Porter’s Five Forces Analysis
11.1 Bargaining Power of Buyers
11.2 Bargaining Power of Suppliers
11.3 Degree of Rivalry
11.4 Threat of New Entrants
11.5 Threat of Substitutes
12. SWOT Analysis
12.1 Strength
12.2 Weakness
12.3 Opportunity
12.4 Threat
13. Key Players Analysis
13.1 Abbott Laboratories
13.1.1 Overviews
13.1.2 Key Person
13.1.3 Recent Developments
13.1.4 Revenue
13.2 Boston Scientific Corporation
13.2.1 Overviews
13.2.2 Key Person
13.2.3 Recent Developments
13.2.4 Revenue
13.3 Edwards Lifesciences
13.3.1 Overviews
13.3.2 Key Person
13.3.3 Recent Developments
13.3.4 Revenue
13.4 Cardinal Health Inc.
13.4.1 Overviews
13.4.2 Key Person
13.4.3 Recent Developments
13.4.4 Revenue
13.5 Medtronic PLC
13.5.1 Overviews
13.5.2 Key Person
13.5.3 Recent Developments
13.5.4 Revenue
13.6 GE Healthcare
13.6.1 Overviews
13.6.2 Key Person
13.6.3 Recent Developments
13.6.4 Revenue
13.7 LivaNova PLC
13.7.1 Overviews
13.7.2 Key Person
13.7.3 Recent Developments
13.7.4 Revenue
13.8 Terumo Corporation
13.8.1 Overviews
13.8.2 Key Person
13.8.3 Recent Developments
13.8.4 Revenue
Reach out to us
Call us on
USA: +1-478-202-3244
INDIA: +91-120-421-9822
Drop us an email at
info@renub.com