China in Vitro Diagnostics Market, Size, Forecast 2024-2030, Industry Trends, Growth, Share, Companies Analysis
Buy NowChina in Vitro Diagnostics Market Outlook
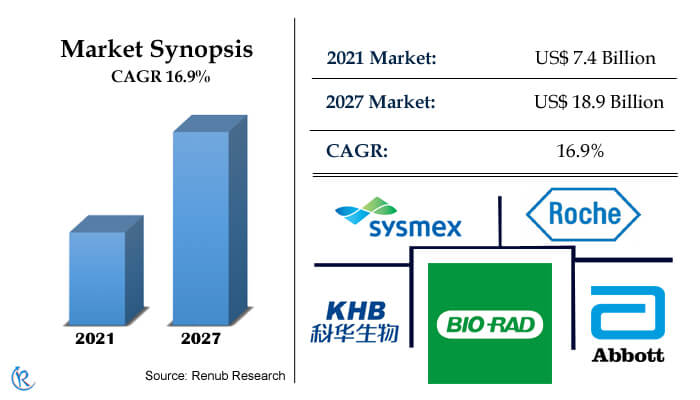
China In-vitro diagnostics (IVD) Market is central to the provision of healthcare globally and is estimated to be US$ 18.9 Billion in 2027. Besides, China is the largest clinical laboratory market in Asia and one of the world's fastest-growing medical realms. Remarkably, over the past years, the rate of Chinese economic growth has been impressive, setting lucrative growth in GDP per year. In addition, the Chinese IVD landscape has historically been controlled by large international providers, with few domestic instrumentation and assay suppliers. Further, looking for a change, the start-up company sees the evolution of diagnostic platforms and assays presenting rapid detection for a wide range of blood-based markers.
China In-Vitro Diagnostics Industry is expanding with double-digit CAGR of 16.9% during 2021-2027
The Chinese IVDs industry is growing for years and has a significant global research and production base. In China, there is a solid clinical demand for the continuous development of IVD enterprises. However, new diagnostic requirements are constantly emerging, requiring clinical laboratories to carry out further testing projects and IVD enterprises to devise new technologies and products. Further, with the improved living standards of the Chinese people and the aging speed of the Chinese population, the demand for family health management is increasing; this avenue will thus become an important growth point for in vitro diagnostics enterprises.
How Coronavirus Benefited China In-Vitro Diagnostics Market Growth Trends
COVID–19 has further accelerated the growth of In-vitro diagnostics industry in China. As China has maintained the zero COVID policy, so in order to achieve that large number of PCR testing and Rapid Antigen tests needs to be performed. Due to COVID variants like Alpha, Beta, Gamma Delta, Delta Plus, and recently Omnicorn, the PCR test and Rapid Antigen tests will keep on happening in huge numbers. According to the Renub Research, the China In-Vitro Diagnostics Market Size was US$ 7.4 Billion in 2021.
Molecular Diagnostics Segment Registers Strong Growth in the Chinese in Vitro Diagnostics Market
In our report, By Segments - China in Vitro Diagnostics Market has been categorized into clinical chemistry, Immunoassay, Molecular Diagnostic, Microbiology, Haematology, and Self-Monitoring of Blood Glucose (SMBG), Point of Care Testing (POCT), and Coagulation. In IVD, one of the most valuable advances has been in the form of molecular diagnostic tools. As per our analysis, the polymerase chain reaction is the most conventional forefront of molecular diagnostics.
Besides, the real-time PCR products simultaneously detect viruses, bacteria, fungi, and parasites, allowing molecular laboratories to lower costs and drive better outcomes in molecular diagnostics. Remarkably, molecular diagnostic tests are used to detect specific sequences in DNA or RNA (including single nucleotide polymorphism (SNP), deletions, rearrangements, insertions, and others) that may or may not be associated with any disease.
Key Players in the Chinese IVD Market
Major international IVD companies already have a substantial presence in the Chinese market and represent potential competitive barriers to prospective market entrants. The key players include Roche Diagnostics, Sysmex Corporation, Bio-Rad Laboratories Inc., Shanghai Kehua Bio-Engineering Co. Ltd., Abbott Laboratories, Danaher Corporation, and BioMerieux SA.
The companies enjoy significantly greater financial resources to afford costs associated with product certification, clinical trials, and agent representation. Additionally, these companies can make the acquisitions necessary to establish direct distribution and local manufacturing operations.
Renub Research report titled “China In–Vitro Diagnostics (IVD) Market, By Segment [Clinical Chemistry, Immunoassay, Molecular Diagnostic, Microbiology, Hematology, Self-Monitoring of Blood Glucose (SMBG), Point of Care Testing (POCT), Coagulation], Companies (Roche Diagnostics, Sysmex Corporation, Bio-Rad Laboratories Inc., Shanghai Kehua Bio-Engineering Co. Ltd., Abbott Laboratories, Danaher Corporation, and BioMerieux SA)” studies the China IVD Industry.
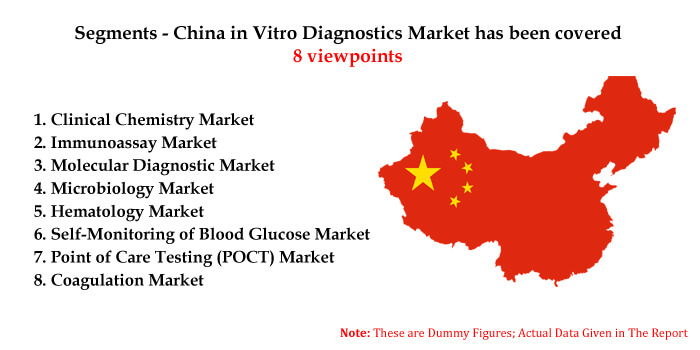
Segments - China in Vitro Diagnostics Market has been covered 8 viewpoints:
1. Clinical Chemistry Market
2. Immunoassay Market
3. Molecular Diagnostic Market
4. Microbiology Market
5. Hematology Market
6. Self-Monitoring of Blood Glucose (SMBG) Market
7. Point of Care Testing (POCT) Market
8. Coagulation Market
Key Players covered in the report which has been studied from 3 viewpoints:
• Overview
• Initiatives & Recent Developments
• Revenue
Company Analysis:
1. Roche Diagnostics
2. Sysmex Corporation
3. Bio-Rad Laboratories Inc.
4. Shanghai Kehua Bio-Engineering Co. Ltd.
5. Abbott Laboratories
6. Danaher Corporation
7. BioMerieux SA
Report Details:
| Report Features | Details |
| Base Year | 2021 |
| Historical Period | 2016 - 2021 |
| Forecast Period | 2022-2027 |
| Market | US$ Billion |
| Segment Covered | Clinical Chemistry, Immunoassay, Molecular Diagnostic, Microbiology, Hematology, Self-Monitoring of Blood Glucose (SMBG), Point of Care Testing (POCT), Coagulation |
| Companies Covered | Roche Diagnostics, Sysmex Corporation, Bio-Rad Laboratories Inc., Shanghai Kehua Bio-Engineering Co. Ltd., Abbott Laboratories, Danaher Corporation, and BioMerieux SA |
| Customization Scope | 20% Free Customization |
| Post-Sale Analyst Support | 1 Year (52 Weeks) |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on request) |
Frequently Asked Questions:
- What is the study period of China IVD market?
- What is the growth rate of China In-vitro Diagnostics Market?
- What will drive the market size of China In-vitro Diagnostics Market globally?
- Who are the key players in China In-vitro Diagnostics Market?
- How will service providers contribute to the. China In-vitro Diagnostics sector?
- How did the ongoing coronavirus (COVID-19) pandemic impact the China In-vitro Diagnostics Industry growth?
1. Introduction
2. Research Methodology
3. Executive Summary
4. Market Dynamics
4.1 Growth Drivers
4.2 Challenges
5. China In-Vitro Diagnostics Market
6. Market Share -China in Vitro Diagnostics
6.1 By Segment
7. Segments - China in Vitro Diagnostics Market
7.1 Clinical Chemistry Market
7.2 Immunoassay Market
7.3 Molecular Diagnostic Market
7.4 Microbiology Market
7.5 Hematology Market
7.6 Self-Monitoring of Blood Glucose (SMBG) Market
7.7 Point of Care Testing (POCT) Market
7.8 Coagulation Market
8. Development Environment of Chinese IVD Industry
8.1 Healthcare Reforms
8.2 Improving Quality at Grass-Root Level
8.3 Embracing Technology
9. China: Health Insurance and Reimbursement Policies
9.1 Health Insurance System
9.1.1 Basic Medical Insurance
9.1.2 Insurance for Other Groups
9.1.3 Rural Medical Insurance
9.1.4 Private Health Insurance
9.2 Reimbursement Rules
9.2.1 Device Reimbursement
9.2.2 Reimbursement for In-Vitro Diagnostics (IVDs)
10. Registration of In Vitro Diagnostic Reagents in China
10.1 Registration and Filing
10.2 Filing Obligation for Clinical Trials
10.3 Clinical Trial Institutions
10.4 Elimination of IVD Loophole for Research
10.5 Change of Manufacturing Address
10.6 Change of Main Supplier of an Antigen or Antibody
10.7 Update on China In-Vitro Diagnostics Registration
10.7.1 IVD Product Registration in China
10.7.2 China IVD Type Testing Process
10.7.3 Clinical Trials for IVD Products in China
11. Medical Devices and Reagents Class Registration in China
11.1 Process of Medical Device Registration in China
11.2 Classification of In-vitro Diagnostic Reagents in China
11.2.1 Class III: Highest Risk
11.2.2 Class II: Medium Risk
11.2.3 Class I: Lower Risk
11.3 China In-Vitro Diagnostics Reagents
11.3.1 Blood Screening Reagents — Drug Administration
11.3.2 Varieties of Blood Screening Reagents
11.3.3 Blood Screening Reagent Test
11.3.4 Nucleic Acid Detection Kits for Blood Screening
11.3.5 Radioactive Reagents — Drug Administration
11.3.6 Diagnostic Reagents - Medical Devices Management
12. Porters Five Forces
12.1 Overview
12.2 Bargaining Power of Buyers
12.3 Bargaining Power of Suppliers
12.4 Degree of Competition
12.5 Threat of New Entrants
12.6 Threat of Substitutes
13. Company Analysis
13.1 Roche Diagnostics
13.1.1 Overviews
13.1.2 Recent Developments
13.1.3 Revenues
13.2 Sysmex Corporation
13.2.1 Overviews
13.2.2 Recent Developments
13.2.3 Revenues
13.3 Bio-Rad Laboratories Inc.
13.3.1 Overviews
13.3.2 Recent Developments
13.3.3 Revenues
13.4 Shanghai Kehua Bio-Engineering Co. Ltd.
13.4.1 Overviews
13.4.2 Recent Developments
13.4.3 Revenues
13.5 Abbott Laboratories
13.5.1 Overviews
13.5.2 Recent Developments
13.5.3 Revenues
13.6 Danaher Corporation
13.6.1 Overviews
13.6.2 Recent Developments
13.6.3 Revenues
13.7 BioMerieux SA
13.7.1 Overviews
13.7.2 Recent Developments
13.7.3 Revenues
14. Profiles of Select Private Clinical Labs and Diagnostic Services Companies
14.1 Zhejiang Di’an Diagnostics Technology Co., Ltd.
14.1.1 Products and Services Offered by Di’an
14.2 ADICON Clinical Laboratories (Privately held)
14.2.1 Products and Services Offered by ADICON
14.3 Guangzhou Kingmed Diagnostics Center Co. Ltd.
14.3.1 Products and Services Offered by Kingmed
14.4 Kindstar Global (Privately held)
14.4.1 Products and Services Offered by Kindstar
14.5 BGI-Shenzhen
14.5.1 BGI’s Innovative Approach
14.6 OriGene Technologies
14.6.1 Products and Services Offered by OriGene
List Of Figures:
Figure-01: China In-Vitro Diagnostics Market (Million US$), 2016 – 2021
Figure-02: Forecast for – China In-Vitro Diagnostics Market (Million US$), 2022 – 2027
Figure-03: Segments – Clinical Chemistry Market (Million US$), 2016 – 2021
Figure-04: Segments – Forecast for Clinical Chemistry Market (Million US$), 2022 – 2027
Figure-05: Segments – Immunoassay Market (Million US$), 2016 – 2021
Figure-06: Segments – Forecast for Immunoassay Market (Million US$), 2022 – 2027
Figure-07: Segments – Molecular Diagnostic Market (Million US$), 2016 – 2021
Figure-08: Segments – Forecast for Molecular Diagnostic Market (Million US$), 2022 – 2027
Figure-09: Segments – Microbiology Market (Million US$), 2016 – 2021
Figure-10: Segments – Forecast for Microbiology Market (Million US$), 2022 – 2027
Figure-11: Segments – Hematology Market (Million US$), 2016 – 2021
Figure-12: Segments – Forecast for Hematology Market (Million US$), 2022 – 2027
Figure-13: Segments – Self-Monitoring of Blood Glucose (SMBG) Market (Million US$), 2016 – 2021
Figure-14: Segments – Forecast for Self-Monitoring of Blood Glucose (SMBG) Market (Million US$), 2022 – 2027
Figure-15: Segments – Point of Care Testing (POCT) Market (Million US$), 2016 – 2021
Figure-16: Segments – Forecast for Point of Care Testing (POCT) Market (Million US$), 2022 – 2027
Figure-17: Segments – Coagulation Market (Million US$), 2016 – 2021
Figure-18: Segments – Forecast for Coagulation Market (Million US$), 2022 – 2027
Figure-19: Roche Diagnostics – Global Revenue (Million US$), 2016 – 2021
Figure-20: Roche Diagnostics – Forecast for Global Revenue (Million US$), 2022 – 2027
Figure-21: Sysmex Corporation – Global Revenue (Million US$), 2016 – 2021
Figure-22: Sysmex Corporation – Forecast for Global Revenue (Million US$), 2022 – 2027
Figure-23: Bio-Rad Laboratories Inc. – Global Revenue (Million US$), 2016 – 2021
Figure-24: Bio-Rad Laboratories Inc. – Forecast for Global Revenue (Million US$), 2022 – 2027
Figure-25: Shanghai Kehua Bio-Engineering Co. Ltd. – Global Revenue (Million US$), 2016 – 2021
Figure-26: Shanghai Kehua Bio-Engineering Co. Ltd. – Forecast for Global Revenue (Million US$), 2022 – 2027
Figure-27: Abbott Laboratories – Global Revenue (Million US$), 2016 – 2021
Figure-28: Abbott Laboratories – Forecast for Global Revenue (Million US$), 2022 – 2027
Figure-29: Danaher Corporation – Global Revenue (Million US$), 2016 – 2021
Figure-30: Danaher Corporation – Forecast for Global Revenue (Million US$), 2022 – 2027
Figure-31: BioMerieux SA – Global Revenue (Million US$), 2016 – 2021
Figure-32: BioMerieux SA – Forecast for Global Revenue (Million US$), 2022 – 2027
List Of Tables:
Table-01: China – In-Vitro Diagnostics Market Share by Segment (Percent), 2016 – 2021
Table-02: China – Forecast for In-Vitro Diagnostics Market Share by Segment (Percent), 2022 – 2027
Reach out to us
Call us on
USA: +1-478-202-3244
INDIA: +91-120-421-9822
Drop us an email at
info@renub.com