Alzheimer's Drugs Market, Global Forecast 2023-2027, Industry Trends, Growth, Insight, Impact of Inflation, Company Analysis
Alzheimer's Drugs Market Outlook
Global Alzheimer's Drugs Market is forecasted to be around USD 7.48 Billion by 2027, according to Renub Research. Alzheimer's disease is a growing brain disorder that, with time, destroys retention and reasoning skills daily. As of know we do not know any reason for the cause Alzheimer disease. Instead, the disease is caused by genetic, lifestyle, and environmental components that influence the brain over time. In older people, Alzheimer disease is the most common source of dementia. According to the World Health Organization (WHO), Alzheimer's disease contributes to 60-70% of dementia cases.
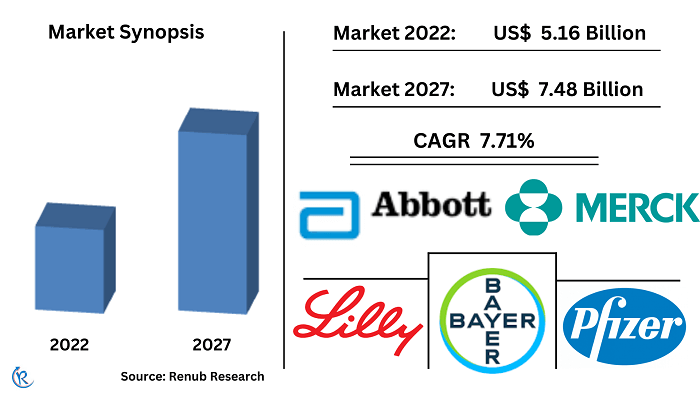
Worldwide Alzheimer's Drugs Market is expected to grow at a CAGR of 7.71% in the forecast period 2022 to 2027
The increase in cases of Alzheimer's disease in the elderly population, the rising generality of chronic diseases, the rise in the government spending on healthcare for improving R&D activities, and a quick rise in the number of drugs, in line, for approval in the coming years, are some of the factors that will contribute significantly to the growth of the Alzheimer's disease drug market.
Nevertheless, the rise in the expenses related to research and development and the ongoing non-performance, unreliability, and disruption of the medical trials of Alzheimer's medicines, restrict the growth of the Alzheimer's disease market.
Donepezil Drug Market will grow more in Forecast Period
Based on Drug class, the Alzheimer's disease drug market is segmented into; Donepezil, Galantamine, Rivastigmine, Memantine, and Others. The donepezil segment has dominated the market share. Although donepezil does not cure dementia, it is known to treat symptoms and help with some forms of dementia. The main reason for the growth of this segment is the best pharmacological treatment options it provides in terms of mental improvement. Donepezil has a response rate of 40%-58%, with a side-effect rate of 6%-13%.
The galantamine segment is also expected to grow in the forecast period. Galantamine improves the mental performance of individuals with Alzheimer's, other dementia-causing disorders, and age-related memory loss by vascular diseases. As per this research report, Globally Alzheimer's Drugs Market was valued at US$ 5.16 Billion in 2022.
Hospital Sector will Capture more Revenue in Upcoming Years
The End-user segment is bifurcated into; Hospitals, Clinics, Nursing homes, and Homecare settings. The hospital segment dominated the market share, owing to the growth in the hospitalization of the older population and Alzheimer's patients. As per the Alzheimer's Association, it is estimated that there are 518 hospitalizations per 1,000 Medicare receivers in elderly citizens having Alzheimer's or other dementias, in contrast to 234 hospitalizations per 1,000 Medicare receivers in the absence of these diseases. The homecare setting segment will see significant growth in the forecast period. Homecare setting has emerged as an alternative to a hospital stay, as it is a less expensive means of treatment.
North America holds Largest Market Share in near Future
Based on region, the Alzheimer's disease market is divided into; North America, Europe, Asia-Pacific, and the Rest of the World. North American region's growth can be credited to the rise in the geriatric population and the increase in the research activities for treating Alzheimer's disease. As per the Alzheimer's Association, in 2016, around 5.4 million people Americans of every age had Alzheimer's disease. One in nine people older than and aged 65 have Alzheimer's disease. The quantity of people aged 65 and above influenced by Alzheimer's disease is said to nearly triple, from 5.2 million to an anticipated 13.8 million, by 2050.
The Asia-Pacific region is expected to show significant growth during the forecast period. The reason for this growth is the increasing understanding among the common people regarding healthcare and the rise in research projects for developing medicines to cure Alzheimer's disease. In addition, the increase in the elderly population in various East-Asian countries is also anticipated to add to the growth of the region's market. According to the World Ageing report, as of 2019, Japan had 35.5 million people over 65, and the number is expected to be around 37.3 million by 2030.
Key Players Analysis
According to our report, the key players in the market are Sanofi SA, F. Hoffmann-La Roche Ltd., Pfizer, Inc., Abbott Laboratories, Bayer, Merck & Co., Boehringer Ingelheim GmbH, GlaxoSmithKline Plc., Novartis, Eli Lilly and Company, and Teva Pharmaceuticals. In addition, investments in R&D activities and new product introductions in the market are some of the plans undertaken by the companies to obtain a more significant market share. For instance, in May 2018, Eli Lilly and Company and AC Immune joined forces to create a new treatment for Alzheimer's disease using AC Immune's Morphomer platform.
Renub Research report titled “Alzheimer’s Drug Market Global Forecast by Drug Class (Donepezil, Galantamine, Rivastigmine, Memantine, and Others), End-user (Hospitals, Clinics, Nursing homes, and Home care centres), Regions (North America, Europe, Asia-Pacific, and Rest of the World), Company (Sanofi SA, F. Hoffman-La Roche Ltd., Pfizer Inc., Abbott Laboratories, Bayer, Merck & Co., Boehringer Ingelheim GmbH, GlaxoSmithKline Plc., Novartis, Eli Lilly and Company, and Teva Pharmaceuticals) Global Analysis” studies the global Alzheimer’s Drug Industry.
Product – Alzheimer’s Drug Market breakup from 5 viewpoints:
1. Donepezil
2. Galantamine
3. Rivastigmine
4. Memantine
5. Others
End User – Alzheimer’s Drug Market breakup from 4 viewpoints:
1. Hospitals
2. Clinics
3. Nursing homes
4. Homecare Centres
Region – Alzheimer’s Drug Market breakup from 4 Regions:
1. North America
2. Europe
3. Asia-Pacific
4. Rest of the World
All key players have been covered from 3 viewpoints:
1. Overview
2. Strategy
3. Financial Insight
Company Analysis:
1. Sanofi SA
2. F. Hoffmann-La Roche Ltd.
3. Pfizer, Inc.
4. Abbott Laboratories
5. Bayer
6. Merck & Co.
7. Boehringer Ingelheim GmbH
8. GlaxoSmithKline Plc.
9. Novartis
10. Eli Lilly and Company
11. Teva Pharmaceuticals
Report Details:
| Report Features | Details |
| Base Year | 2022 |
| Historical Period | 2018 - 2022 |
| Forecast Period | 2023 - 2027 |
| Market | US$ Billion |
| Segment Covered | Drug Class, End-User, and Region |
| Region Covered | North America, Europe, Asia Pacific and Rest of World |
| Companies Covered | Sanofi SA, F. Hoffman-La Roche Ltd., Pfizer Inc., Abbott Laboratories, Bayer, Merck & Co., Boehringer Ingelheim GmbH, GlaxoSmithKline Plc., Novartis, Eli Lilly and Company, and Teva Pharmaceuticals |
| Customization Scope | 20% Free Customization |
| Post-Sale Analyst Support | 1 Year (52 Weeks) |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on request) |
1. Introduction
2. Research Methodology
3. Executive Summary
4. Market Dynamics
4.1 Growth Drivers
4.2 Challenges
5. Global Alzheimer Drugs Market
6. Market Share – Alzheimer Drugs Market
6.1 By Drug Class
6.2 By End User
6.3 By Region
7. Drugs Class – Alzheimer’s Drug Market
7.1 Donepezil
7.2 Galantamine
7.3 Rivastigmine
7.4 Memantine
7.5 Others
8. End User – Alzheimer’s Drug Market
8.1 Hospital
8.2 Clinics
8.3 Nursing Home
8.4 Home Care Setting
9. Region – Alzheimer’s Drug Market
9.1 North America
9.2 Europe
9.3 Asia Pacific
9.4 Rest of World
10. Key Players Analysis
10.1 Sanofi SA
10.1.1 Overview
10.1.2 Recent Development
10.1.3 Net Sales
10.2 F. Hoffmann-La Roche Ltd
10.2.1 Overview
10.2.2 Recent Development
10.2.3 Net Sales
10.3 Pfizer Inc
10.3.1 Overview
10.3.2 Recent Development
10.3.3 Net Sales
10.4 Abbott
10.4.1 Overview
10.4.2 Recent Development
10.4.3 Net Sales
10.5 Bayer AG
10.5.1 Overview
10.5.2 Recent Development
10.5.3 Net Sales
10.6 Merck & Co.Inc
10.6.1 Overview
10.6.2 Recent Development
10.6.3 Net Sales
10.7 Boehringer Ingelheim GmbH
10.7.1 Overview
10.7.2 Recent Development
10.7.3 Net Sales
10.8 Glaxosmithkline Plc
10.8.1 Overview
10.8.2 Recent Development
10.8.3 Net Sales
10.9 Novartis AG
10.9.1 Overview
10.9.2 Recent Development
10.9.3 Net Sales
10.10 Eli Lilly and Company
10.10.1 Overview
10.10.2 Recent Development
10.10.3 Net Sales
10.11 Teva Pharmaceutical Industries Ltd
10.11.1 Overview
10.11.2 Recent Development
10.11.3 Net Sales
List of Figures:
Figure-01: Global – Alzheimer Drugs Market (Billion US$), 2018 – 2022
Figure-02: Global – Forecast for Alzheimer Drugs Market (Billion US$), 2023 – 2027
Figure-03: Drugs Class – Donepezil Market (Million US$), 2018 – 2022
Figure-04: Drugs Class – Forecast for Donepezil Market (Million US$), 2023 – 2027
Figure-05: Drugs Class – Galantamine Market (Million US$), 2018 – 2022
Figure-06: Drugs Class – Forecast for Galantamine Market (Million US$), 2023 – 2027
Figure-07: Drugs Class – Rivastigmine Market (Million US$), 2018 – 2022
Figure-08: Drugs Class – Forecast for Rivastigmine Market (Million US$), 2023 – 2027
Figure-09: Drugs Class – Memantine Market (Million US$), 2018 – 2022
Figure-10: Drugs Class – Forecast for Memantine Market (Million US$), 2023 – 2027
Figure-11: Drugs Class – Others Market (Million US$), 2018 – 2022
Figure-12: Drugs Class – Forecast for Others Market (Million US$), 2023 – 2027
Figure-13: End User – Hospital Market (Million US$), 2018 – 2022
Figure-14: End User – Forecast for Hospital Market (Million US$), 2023 – 2027
Figure-15: End User – Clinics Market (Million US$), 2018 – 2022
Figure-16: End User – Forecast for Clinics Market (Million US$), 2023 – 2027
Figure-17: End User – Nursing Home Market (Million US$), 2018 – 2022
Figure-18: End User – Forecast for Nursing Home Market (Million US$), 2023 – 2027
Figure-19: End User – Home Care Setting Market (Million US$), 2018 – 2022
Figure-20: End User – Forecast for Home Care Setting Market (Million US$), 2023 – 2027
Figure-21: North America – Alzheimer Drugs Market (Million US$), 2018 – 2022
Figure-22: North America – Forecast for Alzheimer Drugs Market (Million US$), 2023 – 2027
Figure-23: Europe – Alzheimer Drugs Market (Million US$), 2018 – 2022
Figure-24: Europe – Forecast for Alzheimer Drugs Market (Million US$), 2023 – 2027
Figure-25: Asia Pacific – Alzheimer Drugs Market (Million US$), 2018 – 2022
Figure-26: Asia Pacific – Forecast for Alzheimer Drugs Market (Million US$), 2023 – 2027
Figure-27: Rest of World – Alzheimer Drugs Market (Million US$), 2018 – 2022
Figure-28: Rest of World – Forecast for Alzheimer Drugs Market (Million US$), 2023 – 2027
Figure-29: Sanofi SA – Global Revenue (Billion US$), 2018 – 2022
Figure-30: Sanofi SA – Forecast for Global Revenue (Billion US$), 2023 – 2027
Figure-31: F. Hoffmann-La Roche Ltd – Global Revenue (Billion US$), 2018 – 2022
Figure-32: F. Hoffmann-La Roche Ltd – Forecast for Global Revenue (Billion US$), 2023 – 2027
Figure-33: Pfizer Inc – Global Revenue (Billion US$), 2018 – 2022
Figure-34: Pfizer Inc – Forecast for Global Revenue (Billion US$), 2023 – 2027
Figure-35: Abbott – Global Revenue (Billion US$), 2018 – 2022
Figure-36: Abbott – Forecast for Global Revenue (Billion US$), 2023 – 2027
Figure-37: Bayer AG – Global Revenue (Billion US$), 2018 – 2022
Figure-38: Bayer AG – Forecast for Global Revenue (Billion US$), 2023 – 2027
Figure-39: Merck & Co.Inc – Global Revenue (Billion US$), 2018 – 2022
Figure-40: Merck & Co.Inc – Forecast for Global Revenue (Billion US$), 2023 – 2027
Figure-41: Boehringer Ingelheim GmbH – Global Revenue (Billion US$), 2018 – 2022
Figure-42: Boehringer Ingelheim GmbH – Forecast for Global Revenue (Billion US$), 2023 – 2027
Figure-43: Glaxosmithkline Plc – Global Revenue (Billion US$), 2018 – 2022
Figure-44: Glaxosmithkline Plc – Forecast for Global Revenue (Billion US$), 2023 – 2027
Figure-45: Novartis AG – Global Revenue (Billion US$), 2018 – 2022
Figure-46: Novartis AG – Forecast for Global Revenue (Billion US$), 2023 – 2027
Figure-47: Eli Lilly and Company – Global Revenue (Billion US$), 2018 – 2022
Figure-48: Eli Lilly and Company – Forecast for Global Revenue (Billion US$), 2023 – 2027
Figure-49: Teva Pharmaceutical Industries Ltd – Global Revenue (Billion US$), 2018 – 2022
Figure-50: Teva Pharmaceutical Industries Ltd – Forecast for Global Revenue (Billion US$), 2023 – 2027
List of Tables:
Table-01: Global – Alzheimer Drugs Market Share by Drug Class (Percent), 2018 – 2022
Table-02: Global – Forecast for Alzheimer Drugs Market Share by Drug Class (Percent), 2023 – 2027
Table-03: Global – Alzheimer Drugs Market Share by End User (Percent), 2018 – 2022
Table-04: Global – Forecast for Alzheimer Drugs Market Share by End User (Percent), 2023 – 2027
Table-05: Global – Alzheimer Drugs Market Share by Region (Percent), 2018 – 2022
Table-06: Global – Forecast for Alzheimer Drugs Market Share by Region (Percent), 2023 – 2027
Reach out to us
Call us on
USA: +1-678-302-0700
INDIA: +91-120-421-9822
Drop us an email at
info@renub.com


