Global Medical Writing Market – Trends & Forecast 2025–2033
Buy NowMedical Writing Market Growth, Segments & Forecast 2025–2033 | Renub Research
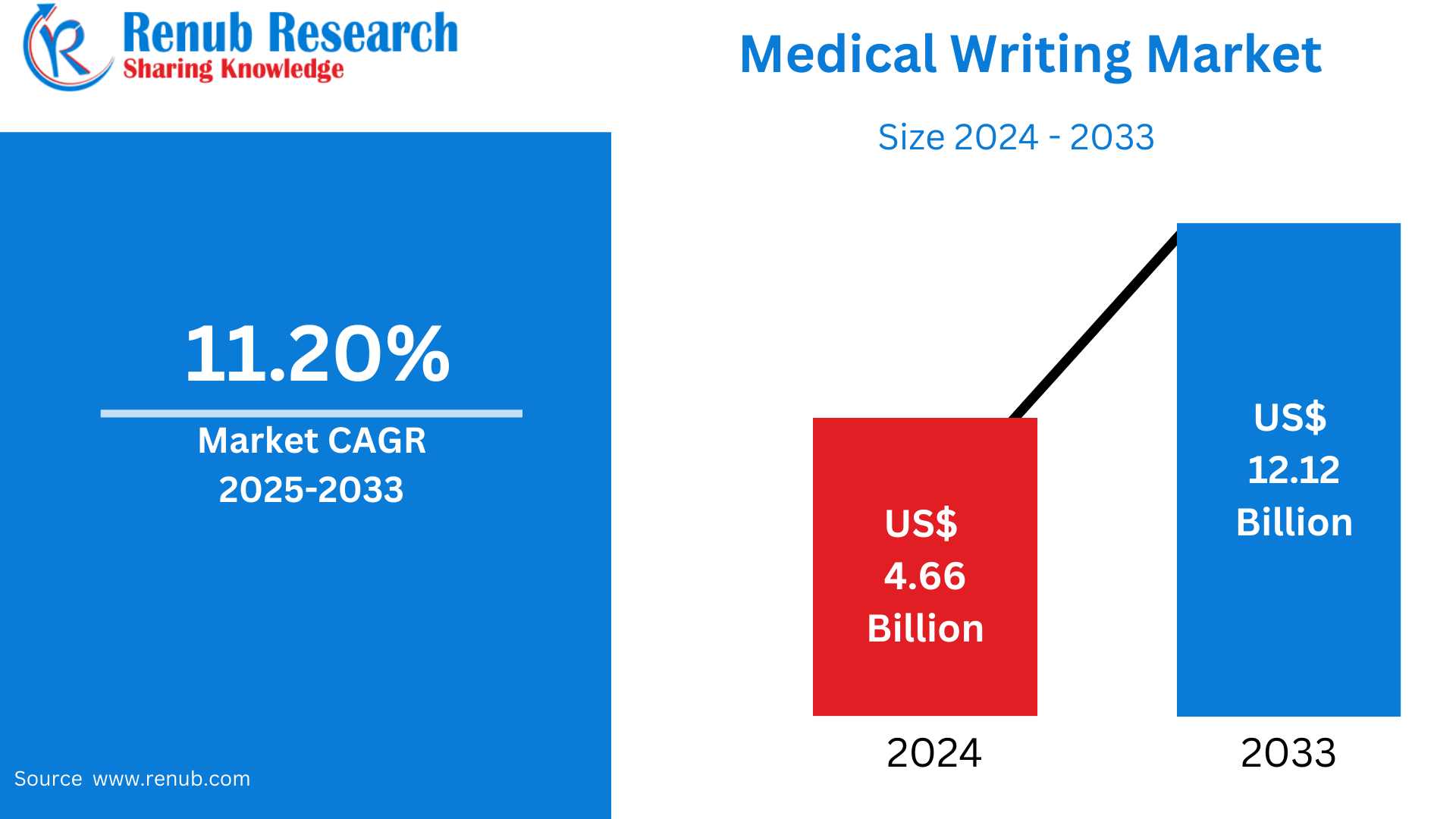
Medical Writing Market is expected to reach US$ 12.12 billion by 2033 from US$ 4.66 billion in 2024, with a CAGR of 11.20% from 2025 to 2033. The expansion of the pharmaceutical and biotechnology industries, the necessity of increased regulatory documentation, growth in clinical trials, the greater focus on drug compliance and safety, technological innovations, trends towards outsourcing, increase in personalized medicine, and health communication are all fueling medical writing industry growth.
Global Medical Writing Market Report by Type (Clinical Writing, Regulatory Writing, Scientific Writing, Others), Application (Medical Journalism, Medical Education, Medico Marketing, Others), End Use (Contract Research Organizations & Others, Medical Device/Pharmaceutical & Biotechnology Companies), Countries and Company Analysis 2025-2033.
Global Medical Writing Industry Overview
Scientific papers concerning medicine, health care, and drug development well written is referred to as medical writing. Journal articles, educational materials, marketing materials, and regulatory documents (like clinical trial reports and regulatory submissions) are all part of it. For the good of regulatory agencies, medical professionals, and the general public, medical writers translate complex clinical and scientific realities into comprehensible, precise, and compliant documents. They collaborate with marketers, researchers, and regulatory agencies to ensure the clarity and integrity of science. It is essential to be familiar with drug development processes, medical jargon, and regulatory affairs. Medical writing is vital to the healthcare industry due to the escalating medical research complexity and global legislation.
Several key factors drive the medical writing business. Demand is heightened by the number of clinical trials and medicinal licenses escalating and requiring much documentation. The increasing biotechnology, pharmaceutical, and medical device industries all contribute to market growth. The demand for specialized writing services is driven by increasing regulatory demands and the need for compliance documents. Traditional publishing models are replaced by online publications by the increasing scholarly publications, digital healthcare communication, and personalized medicine, driving the demand for content up even more. Writing processes are also simplified through advances in technology and AI technologies, which fuel market expansion. This growth is further boosted through aging populations as well as emerging markets.
Growth Drivers for the Medical Writing Market
Rising number of clinical trials and medicinal approvals
The medical writing industry is impacted by a number of critical factors. The increasing number of medicinal approvals and clinical trials, which require a tremendous amount of paperwork, boost demand. Expanding biotechnology, pharmaceutical, and medical device industries all fuel market growth. Increasing regulatory needs and the need for compliance documents are fueled by growing requirements. The demand for skilled medical writers is boosted by the global phenomenon of offshoring non-core activities. The growth in scholarly publishing, electronic healthcare communication, and customized medicine also boosts the demand for content. Writing processes are similarly streamlined by technological advancements and AI technologies, enhancing market growth. This trend is further boosted by aging populations and growing markets. The focus of the industry to penetrate high-growth markets is reflected in this strategic push to enhance its presence in the Asia Pacific market. These expansions serve to reflect the way the medical writing market is directly spurred by growth in the biotech and pharmaceutical industries.
Rising R&D Activities
The medical writing market is largely prompted by increased R&D spending by biotechnology, pharmaceutical, and medical device companies. The need for accurate, acceptable documentation, such as clinical research reports, trial procedures, and scientific publications, is growing as R&D operations evolve. Companies seek experienced medical writers to facilitate regulatory submissions and ensure that detailed data are properly communicated. FlexSteel Technologies Holdings, Inc. was purchased by Cactus Communications in January 2023 for $621 million upfront with an additional potential $75 million earn-out, a significant development reflecting market momentum. The acquisition highlights Cactus' desire to grow and increase its scientific communication and medical writing expertise across broader industry sectors, despite FlexSteel focusing on spoolable pipe technologies.
Globalization of Drug Development
The demand for medical writing services is increasing exponentially due to the globalisation of drug development. Pharmaceutical and biotech companies have to adhere to different regulatory requirements and submit uniform, top-quality documentation across numerous jurisdictions in which they perform clinical trials. Due to this, there is tremendous demand for trained medical writers who can navigate global regulatory landscapes and ensure uniform communication. The demand for experienced writers is also driven by the localisation of the language of documents and cultural adaptation. In line with this trend, BioMed Solutions launched an innovative medical writing training program in April 2023 to equip professionals with the expertise they require in order to thrive in this emerging business area. This reflects the increasing importance of international exposure to the field of medical writing.
Challenges in the Medical Writing Market
Maintaining Consistency and Quality
In the market for medical writing, maintaining consistency and quality is a significant difficulty. There is a greater chance of data, tone, and terminology inconsistencies when there are numerous stakeholders involved, including clinicians, researchers, and regulatory specialists. The homogeneity of sections is further complicated by large, multi-author projects. Quality assurance is a laborious but crucial component of medical writing since it takes careful editing, version control, and adherence to templates and procedures to ensure high-quality output that satisfies stringent regulatory and scientific standards.
Shortage of Skilled Medical Writers
The medical writing sector has a major challenge: a lack of qualified medical writers. There is an urgent need for people with both scientific understanding and regulatory writing expertise due to the increasing need for clinical trials, scientific publications, and regulatory submissions. Finding suitable authors is challenging due to the specialist nature of the task and the ongoing changes in medical legislation. Increased competition for qualified individuals and project delays may result from this skills shortage.
United States Medical Writing Market
The expanding pharmaceutical and healthcare industries are driving the demand for medical writing in the United States. The large number of clinical trials, especially in therapeutic fields like neurology, cardiology, and oncology, is a significant contributing element. As of July 2022, around 10,548 clinical studies were in Phase III for cancer, 6,349 for cardiology, and 947 for neurology, according to clinicaltrials.gov. Research-related documentation increases as a result of more clinical studies, especially Phase III trials, necessitating accurate and fast medical writing. It is anticipated that over the projection period, the growing need for clinical research reports, scientific publications, and regulatory filings will propel the medical writing market forward, opening doors for both seasoned pros and up-and-coming service providers.
India Medical Writing Market
The burgeoning pharmaceutical, biotechnology, and healthcare industries are driving India's medical writing market's rapid expansion. The demand for medical writing services is greatly increased by India's recognized position as a global center for clinical trials and regulatory submissions. India is a desirable location for outsourcing medical writing duties, such as clinical trial reports, regulatory documentation, and scientific publications, due to its highly qualified specialists and sizable English-speaking workforce. Further strengthening India's competitive edge are affordable services and the use of cutting-edge technologies like artificial intelligence. India's medical writing market is expected to continue expanding in the upcoming years as the nation continues to draw in foreign clientele.
United Kingdom Medical Writing Market
The robust healthcare system in the UK and the rising need for clinical and regulatory documentation are fueling the market for medical writing in the country. Two major reasons driving market expansion are the increase in clinical trials and the requirement for precise, compliance documentation. The category that generates the most revenue is still clinical writing, albeit regulatory writing is also expanding significantly. The UK is a desirable place for both domestic and foreign medical writing services due to its established pharmaceutical and biotechnology industries and highly qualified workforce. This establishes the UK as a major force in the international medical writing market.
United Arab Emirates Medical Writing Market
The United Arab Emirates' (UAE) medical writing market is expanding steadily due to improvements in the country's healthcare system, a rise in clinical research, and an increase in the need for scientific and regulatory documentation. The UAE's dedication to healthcare innovation and its advantageous location as a medical tourism hub both support the market's growth. The need for specialized medical writing services is also being impacted by the uptake of digital health technology and the focus on adhering to global regulatory requirements. The necessity for accurate and thoroughly documented medical material is anticipated to increase as the healthcare industry develops further, making the UAE a major player in the regional medical writing scene.
Medical Writing Market Segments:
Type
- Clinical Writing
- Regulatory Writing
- Scientific Writing
- Others
Application
- Medical Journalism
- Medical Education
- Medico Marketing
- Others
End Use
- Contract Research Organizations & Others
- Medical Device/Pharmaceutical & Biotechnology Companies
Country – Market breakup in 25 viewpoints:
North America
- United States
- Canada
Europe
- France
- Germany
- Italy
- Spain
- United Kingdom
- Belgium
- Netherlands
- Turkey
Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Thailand
- Malaysia
- Indonesia
- New Zealand
Latin America
- Brazil
- Mexico
- Argentina
Middle East & Africa
- South Africa
- Saudi Arabia
- United Arab Emirates
All companies have been covered from 4 viewpoints:
- Company Overview
- Key Persons
- Recent Development & Strategies
- Sales Analysis
Key Players Analysis
- Parexel International Corporation
- Trilogy Writing & Consulting GmBH
- Freyr
- Cactus Communications
- Labcorp Drug Development
- IQVIA Holdings Inc.
- Omics International
- Synchrogenix
Report Details:
| Report Features | Details |
| Base Year |
2024 |
| Historical Period |
2021 - 2024 |
| Forecast Period |
2025 - 2033 |
| Market |
US$ Billion |
| Segment Covered |
Type, Application, End User and Countries |
| Countries Covered |
|
| Companies Covered |
|
| Customization Scope |
20% Free Customization |
| Post-Sale Analyst Support |
1 Year (52 Weeks) |
| Delivery Format |
PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on request) |
Customization Services available
- Analysis of Market Size and Its Segments
- More Company Profiles (Upto 10 without any additional cost):
- Additional Countries (Other than mentioned Countries):
- Region/Country Specific Reports:
- Market Entry Strategy:
- Region-Specific Market Dynamics:
- Regional Market Share Analysis:
- Trade Analysis:
- Production Insights:
- Others Customized Requests:
For more information contact our analysts.
Need More Assistance?
- Talk to our analysts to get more precious information on the current market trends.
- Include more countries and segments and customize the report based on the final requirement.
- Get a competitive advantage in your industry by knowing the report findings and making a positive impact on your revenues and operations.
- Our analysts are always ready to provide more help and pertinent information if you need any additional assistance.
1. Introduction
2. Research Methodology
2.1 Data Source
2.1.1 Primary Sources
2.1.2 Secondary Sources
2.2 Research Approach
2.2.1 Top-Down Approach
2.2.2 Bottom-Up Approach
2.3 Forecast Projection Methodology
3. Executive Summary
4. Market Dynamics
4.1 Growth Drivers
4.2 Challenges
5. Medical Writing Market
5.1 Historical Market Trends
5.2 Market Forecast
6. Medical Writing Market Share Analysis
6.1 By Type
6.2 By Application
6.3 By End Use
6.4 By Countries
7. Type
7.1 Clinical Writing
7.2 Regulatory Writing
7.3 Scientific Writing
7.4 Others
8. Application
8.1 Medical Journalism
8.2 Medical Education
8.3 Medico Marketing
8.4 Others
9. End Use
9.1 Contract Research Organizations & Others
9.2 Medical Device/Pharmaceutical & Biotechnology Companies
10. Countries
10.1 North America
10.1.1 United States
10.1.2 Canada
10.2 Europe
10.2.1 France
10.2.2 Germany
10.2.3 Italy
10.2.4 Spain
10.2.5 United Kingdom
10.2.6 Belgium
10.2.7 Netherlands
10.2.8 Turkey
10.3 Asia Pacific
10.3.1 China
10.3.2 Japan
10.3.3 India
10.3.4 South Korea
10.3.5 Thailand
10.3.6 Malaysia
10.3.7 Indonesia
10.3.8 Australia
10.3.9 New Zealand
10.4 Latin America
10.4.1 Brazil
10.4.2 Mexico
10.4.3 Argentina
10.5 Middle East & Africa
10.5.1 Saudi Arabia
10.5.2 UAE
10.5.3 South Africa
11. Porter’s Five Forces Analysis
11.1 Bargaining Power of Buyers
11.2 Bargaining Power of Suppliers
11.3 Degree of Rivalry
11.4 Threat of New Entrants
11.5 Threat of Substitutes
12. SWOT Analysis
12.1 Strength
12.2 Weakness
12.3 Opportunity
12.4 Threat
13. Key Players Analysis
13.1 Parexel International Corporation
13.1.1 Overview
13.1.2 Key Persons
13.1.3 Recent Development & Strategies
13.1.4 Revenue Analysis
13.2 Trilogy Writing & Consulting GmBH
13.2.1 Overview
13.2.2 Key Persons
13.2.3 Recent Development & Strategies
13.2.4 Revenue Analysis
13.3 Freyr
13.3.1 Overview
13.3.2 Key Persons
13.3.3 Recent Development & Strategies
13.3.4 Revenue Analysis
13.4 Cactus Communications
13.4.1 Overview
13.4.2 Key Persons
13.4.3 Recent Development & Strategies
13.4.4 Revenue Analysis
13.5 Labcorp Drug Development
13.5.1 Overview
13.5.2 Key Persons
13.5.3 Recent Development & Strategies
13.5.4 Revenue Analysis
13.6 IQVIA Holdings Inc.
13.6.1 Overview
13.6.2 Key Persons
13.6.3 Recent Development & Strategies
13.6.4 Revenue Analysis
13.7 Omics International
13.7.1 Overview
13.7.2 Key Persons
13.7.3 Recent Development & Strategies
13.7.4 Revenue Analysis
13.8 Synchrogenix
13.8.1 Overview
13.8.2 Key Persons
13.8.3 Recent Development & Strategies
13.8.4 Revenue Analysis
Reach out to us
Call us on
USA: +1-478-202-3244
INDIA: +91-120-421-9822
Drop us an email at
info@renub.com