CAR T Cell Therapy Market Global Forecast by Regions, Targeted Antigens, Clinical Trials/Study, Companies
Buy NowGet Free Customization in This Report
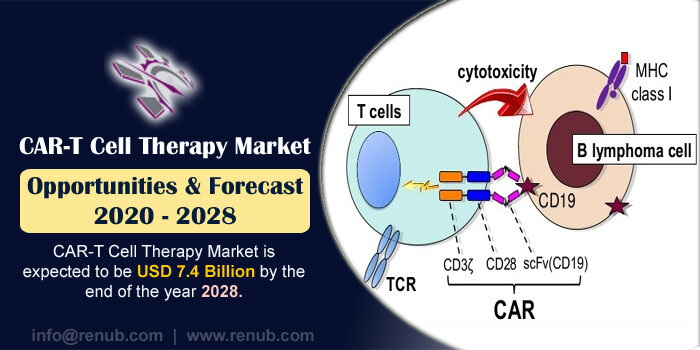
For many years, the support of cancer therapy was chemotherapy, surgery and radiation therapy. However, in recent times, CAR-T cell therapy has been introduced as an incredibly supportive treatment for cancer patients. Since the introduction of chemotherapy, this treatment is one of the most significant breakthroughs. In this therapy, immune cells are collected from patients, and it is modified in the laboratory by doctors. After modification, these immune cells are infused back into the patient as they can easily recognize and kill cancer cells. These infused cells get multiplied and stay in the body as “living drugs.” According to Renub Research analysis, CAR-T Cell Therapy Market is expected to be USD 7.4 Billion by the end of the year 2028.
Growth Factors for CAR-T Cell Therapy
Factors such as growing numbers of cancer in adults and children, and increasing policy initiatives to encourage cell therapy research in cancer, and increasing numbers of clinical trials worldwide are some of the main drivers for the global demand for CAR T cell therapy. The economic scenario in the CAR-T cell therapy industry is very dynamic, and key players compete with each other to gain access to major markets in the United States and Europe. Companies are seeking to secure treatment facilities to increase access for patients to their treatments.
Developments did by Companies in CAR T Cell Therapy
In 2017, a new milestone was set for oncology patients when the FDA approved the first two CD19-targeted known as (Chimeric Antigen Receptor) CAR T cell therapies produced by Novartis and Gilead Sciences known as Kite Pharma in the United States. Such two approvals have helped to improve the global demand for CAR T cell therapy because more companies are searching for this excellent opportunity to reach the marketplace. More than 200 CAR T clinical trials are ongoing or completed in various parts of the world. In 2018, Novartis announced its 33 approved centres in the U.S. and Gilead announced its 28 approved centres for the care of patients. Companies are also coming up with new developments in the field.
Celgene, a global company, is expected to file for approval in USFDA of bb2121 (idecabtagene vicleucel) in myeloma during the first half of 2020 and lisocabtagene maraleucel in lymphoma in the fourth quarter of 2020.
Renub Research latest study report “CAR T Cell Therapy Market Global Forecast by Regions (North America, Europe, Asia Pacific, Latin America, Middle East, Africa), Targeted Antigens (CD19, CD20, GD2, CD22, CD30, CD33, HER2, MESO, EGFRvII, Others), Clinical Trials/Study (CD19, CD20, GD2, CD22, CD30, CD33, HER1, HER2, MESO, EGFRvII), Companies (Novartis, Gilead Sciences (Kite Pharma), Celgene Corporation (Juno Therapeutics), Celyad)” provides a detailed and comprehensive insight of the global CAR T cell therapy market.
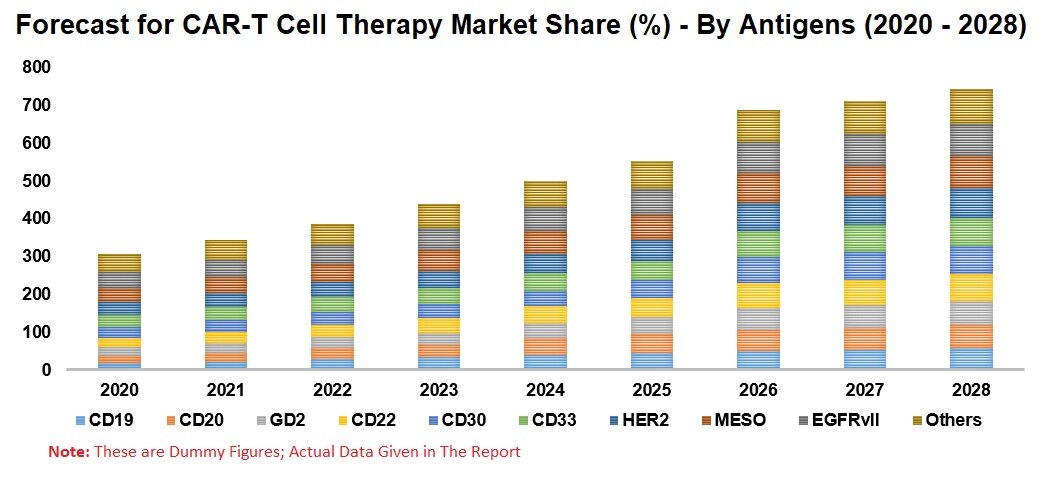
By Antigens – Based on antigens, CAR T Cell Therapy Market is further Segmented into 10 Segments
1. CD19
2. CD20
3. GD2
4. CD22
5. CD30
6. CD33
7. HER2
8. MESO
9. EGFRvII
10. Others
By Region – 6 Regions CAR T Cell Market is studied in this report
1. North America
2. Europe
3. Asia Pacific
4. Latin America
5. Middle East
6. Africa
All the companies have been studied from three points
• Overview
• Initiatives & Recent Developments
• Revenue
Key Companies covered in this report are
• Novartis
• Gilead Sciences (Kite Pharma)
• Celgene Corporation (Juno Therapeutics)
• Celyad
Global – CAR T Cell Therapy Clinical Trials/Study
1. CD19
2. CD20
3. GD2
4. CD22
5. CD30
6. CD33
7. HER1
8. HER2
9. MESO
10. EGFRvII
China CAR T Cells Clinical Trials Details
• By Cities CAR T Cells Clinical Trials
• CD19 Directed CAR T Cells Clinical Trials
• Non-CD19 Directed CAR T Cells Clinical Trials
• Solid Tumors CAR T Cells Clinical Trials
Regulation of CAR T Cell Therapy in 3 Regions is covered in the report
• United States
• European Union
• China
IPO/Investment/Funding/Partnership in CAR T Cell Therapy Market
• Venture Capital Investment
• Initial Public Offerings
• Strategic Partnerships/Deals
• Key CAR T Technology Deals
If the information you seek is not included in the current scope of the study kindly share your specific requirements with our custom research team at info@renub.com
1. Executive Summary
2. Global Chimeric Antigen Receptor (CAR)-T cell Therapy Market
3. Market Share – CAR-T Cell Therapy
3.1 By Geographical Region
3.2 By Targeted Antigens
4. Targeted Antigen Market
4.1 CD19
4.1.1 Introduction
4.1.2 Market Size and Forecast
4.2 CD20
4.2.1 Introduction
4.2.2 Market Size and Forecast
4.3 GD2
4.3.1 Introduction
4.3.2 Market Size and Forecast
4.4 CD22
4.4.1 Introduction
4.4.2 Market Size and Forecast
4.5 CD30
4.5.1 Introduction
4.5.2 Market Size and Forecast
4.6 CD33
4.6.1 Introduction
4.6.2 Market Size and Forecast
4.7 HER2
4.7.1 Introduction
4.7.2 Market Size and Forecast
4.8 Mesothelin (MESO)
4.8.1 Introduction
4.8.2 Market Size and Forecast
4.9 EGFRvIII
4.9.1 Introduction
4.9.2 Market Size and Forecast
4.10 Others
4.10.1 Market Size and Forecast
5. Geographical CAR-T Cell Therapy Market (2016 -2026)
5.1 North America
5.2 Europe
5.3 Asia Pacific
5.4 Latin America
5.5 Middle East
5.6 Africa
6. Global - CAR-T Cell Clinical Trials/Study
6.1 CD19
6.2 CD20
6.3 CD22
6.4 CD30
6.5 CD33
6.6 EGFRvIII
6.7 GD2
6.8 HER1
6.9 HER2
6.10 MESO
7. China CAR-T Cells Clinical Trials Details
7.1 By Cities CAR-T Cells Clinical Trials
7.2 CD19 Directed CAR-T Cells Clinical Trials
7.3 Non-CD19 Directed CAR-T Cells Clinical Trials
7.4 Solid Tumors CAR-T Cells Clinical Trials
8. CAR-T Cell Therapy SWOT Analysis
8.1 Strength
8.2 Weakness
8.3 Opportunities
8.4 Threats
9. Regulation in CAR-T Cell Therapy
9.1 United States
9.2 European Union
9.3 China
10. IPO/Investment/Funding/Partnership in CAR-T Cell Therapy Market
10.1 Venture Capital Investment
10.2 Initial Public Offerings of CAR-T Companies
10.3 CAR-T Companies Strategic Partnerships/Deals
10.4 Key CAR-T Technology Deals
11. Growth Drivers
11.1 FDA Approvals of CAR-T Therapy
11.2 Dramatically Increasing Number of CAR-T Cell Trials Globally
12. Challenges
12.1 Regulatory Challenges
12.2 Very Expensive CAR-T Therapy Treatment
13. Novartis
13.1 Company Overview
13.2 Initiatives
13.2.1 Point 1
13.2.2 Point 2 Kymriah® (tisagenlecleucel), First-in-class CAR-T Therapy from Novartis Received Second FDA Approval
13.2.3 Point 2
13.3 Financial Insight
14. Gilead Sciences (Kite Pharma)
14.1 Company Overview
14.2 Company Initiatives
14.2.1 Point 1
14.2.2 Point 2
14.3 Company Financial Insight
15. Celgene Corporation (Juno Therapeutics)
15.1 Company Overview
15.2 Company Initiatives
15.2.1 Point 1
15.2.2 Point 2
15.3 Company Financial Insight
16. Celyad
16.1 Company Overview
16.2 Company Initiatives
16.2.1 Point 1
16.2.2 Point 2
16.2.3 Point 3
16.3 Financial Insight
List of Figures:
Figure 2‑1: Global – CAR-T Cell Therapy Market (Million US$), 2017 – 2019
Figure 2‑2: Global – Forecast for CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 3‑1: Global – CAR-T Cell Therapy Regional Market Share (Percent), 2017 – 2019
Figure 3‑2: Global – Forecast for CAR-T Cell Therapy Regional Market Share (Percent), 2020 – 2028
Figure 4‑1: Global – CD19 Antigen CAR-T Cell Therapy Market (Million US$), 2017 – 2019
Figure 4‑2: Global – CD19 Antigen CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 4‑3: Global – Forecast for CD20 Antigen CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 4‑4: Global – Forecast for GD2 Antigen CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 4‑5: Global – Forecast for CD22 Antigen CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 4‑6: Global – Forecast for CD30 Antigen CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 4‑7: Global – Forecast for CD33 Antigen CAR-T Cell Therapy Market (Million US$), 2017 – 2028
Figure 4‑8: Global – Forecast for HER2 Antigen CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 4‑9: Global – Forecast for MESO Antigen CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 4‑10: Global – Forecast for EGFRvIII Antigen CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 4‑11: Global – Forecast for Others Antigen CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 5‑1: North America – CAR-T Cell Therapy Market (Million US$), 2017 – 2019
Figure 5‑2: North America – Forecast for CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 5‑3: Europe – CAR-T Cell Therapy Market (Million US$), 2017 – 2019
Figure 5‑4: Europe – Forecast for CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 5‑5: Asia Pacific – CAR-T Cell Therapy Market (Million US$), 2017 – 2019
Figure 5‑6: Asia Pacific – Forecast for CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 5‑7: Latin America – CAR-T Cell Therapy Market (Million US$), 2017 – 2019
Figure 5‑8: Latin America – Forecast for CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 5‑9: Middle East – CAR-T Cell Therapy Market (Million US$), 2017 – 2019
Figure 5‑10: Middle East – Forecast for CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 5‑11: Africa – CAR-T Cell Therapy Market (Million US$), 2017 – 2019
Figure 5‑12: Africa – Forecast for CAR-T Cell Therapy Market (Million US$), 2020 – 2028
Figure 7‑1: China – By Cities CAR-T Cells Clinical Trials (Number), 2017
Figure 11‑1: Global – CAR-T New & Total Clinical Trials (Number), 2012-2017
Figure 11‑2: Global – By Phase CAR-T Clinical Trials (Number), 2017
Figure 13‑1: Novartis – Global Sales (Million US$), 2015 - 2019
Figure 13‑2: Novartis – Forecast for Global Sales (Million US$), 2020 - 2028
Figure 14‑1: Gilead Sciences – Global Sales (Million US$), 2017 - 2019
Figure 14‑2: Gilead Sciences – Forecast for Global Sales (Million US$), 2020 - 2028
Figure 15‑1: Celgene – Global Sales (Million US$), 2013 - 2019
Figure 15‑2: Celgene – Forecast for Global Sales (Million US$), 2020 - 2028
Figure 16‑1: Celyad – Global Sales (Million US$), 2013 - 2019
Figure 16‑2: Celyad – Forecast for Global Sales (Million US$), 2020 - 2028
List of Tables:
Table 3 1: Global – CAR-T Cell Therapy Targeted Antigens Market Share (Percent), 2017 – 2019
Table 3 2: Global – CAR-T Cell Therapy Targeted Antigens Market Share (Percent), 2020 – 2028
Table 6 1: Global – Details of CD19 CAR-T Studies, 2018
Table 6 2: Global – Details of CD20 CAR-T Studies, 2018
Table 6 3: Global – Details of CD22 CAR-T Studies, 2018
Table 6 4: Global – Details of CD30 CAR-T Studies, 2018
Table 6 5: Global – Details of CD33 CAR-T Studies, 2018
Table 6 6: Global – Details of EGFRvIII CAR-T Studies, 2018
Table 6 7: Global – Details of GD2 CAR-T Studies, 2018
Table 6 8: Global – Details of HER1 CAR-T Studies, 2018
Table 6 9: Global – Details of HER2 CAR-T Studies, 2018
Table 6 10: Global – Details of MESO CAR-T Studies, 2018
Table 7 1: China - CD19 Directed CAR-T Cells Clinical Trials, 2017
Table 7 2: China – Non-CD19 Directed CAR-T Cells Clinical Trials, 2017
Table 7 3: China – Solid Tumors CAR-T Cells Clinical Trials, 2017
Table 10 1: Global – Venture Capital Investments (Million US$) in CAR-T Cell Therapy, 2011 – 2017
Table 10 2: Global – Initial Public Offerings (Million US$) in CAR-T Cell Therapy, 2011 – 2016
Table 10 3: Global - CAR-T Companies Strategic Partnerships/Acquisitions, 2012-2017
Table 10 4: Global – Key CAR-T Technology Deals, 2012-2017
Reach out to us
Call us on
USA: +1-478-202-3244
INDIA: +91-120-421-9822
Drop us an email at
info@renub.com